原子和分子中的电子热交换促进了电荷的传递
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
这项工作汇编了近十年来温度依赖化学反应理论的理论进展,引入了第一个有限温度电荷转移模型,预测了化学相互作用过程中的分数电子转移。关键的观点是电子热量驱动电荷转移。通过分析热力学参数,如电子热容量、柔软度和化学势,该框架解释了物质是如何从惰性状态转变为反应状态的,在反应状态下,电子是去相关的,足以实现电荷转移。该模型的一个关键方面是热波动的作用,它控制分子响应函数并促进能量和电荷的同时交换。这个模型被简化为一个简单的关于储层化学势的线性方程。外推时,它支持亲电性指数,增加了一个修正项,并提供了一个更受电子亲和影响的工作公式。这些发现为分析和预测有限温度下的化学相互作用提供了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
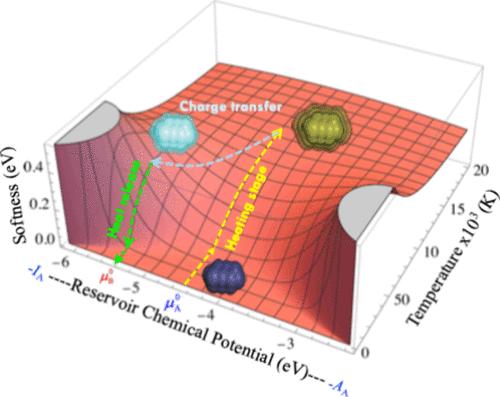
Charge Transfer Is Promoted by Electronic Heat Exchange in Atoms and Molecules
This work compiles almost a decade of theoretical progress in temperature-dependent chemical reactivity theory to introduce the first finite-temperature charge transfer model, predicting fractional electron transfers during chemical interactions. The key insight is that electronic heat drives charge transfer. By analyzing thermodynamic parameters like electronic heat capacity, softness, and chemical potential, the framework explains how species transition from inert to reactive states, where electrons are decorrelated enough to enable charge transfer. A crucial aspect of this model is the role of thermal fluctuations, which governs molecular response functions and facilitates the simultaneous exchange of energy and charge. This model is reduced to a simple linear equation in the chemical potential of the reservoir. When extrapolated, it supports the electrophilicity index, adding a correction term and providing a working formula more influenced by electron affinity. These findings offer new pathways to analyze and predict chemical interactions under the finite temperature regime.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


