IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
为了应对抗菌药耐药性的挑战,我们研究了基于富含赖氨酸和亮氨酸序列的新型抗菌肽。我们通过在序列的 i 和 i+4 位应用碳氢化合物订书钉来稳定其具有膜活性的二级结构。无论订书钉位置如何,订书钉都能提高肽在水环境和脂质环境中的结构稳定性。它还提高了对革兰氏阴性菌和革兰氏阳性菌(包括抗生素耐药菌株)的抗菌效率,最低抑菌浓度(MIC)为 2 至 4 μM(2.5 至 5.5 μg/mL)。钉合肽显示出更强的抗酶降解能力,尤其是在 N 端附近加入钉合肽时。分子动力学模拟揭示了钉书钉如何(i)稳定两性肽的膜活性二级结构和(ii)加速它们的膜插入。我们的研究结果为抗菌肽的设计提供了启示。我们的研究结果表明,对富含赖氨酸和亮氨酸的短序列进行碳氢化合物钉合处理,可为开发更稳定、更有效的抗菌剂提供一条途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
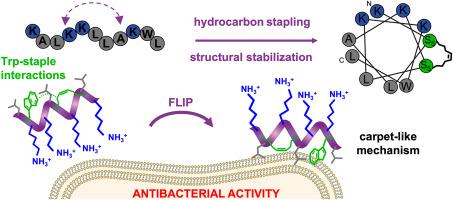
Design, synthesis and evaluation of lysine- and leucine-rich hydrocarbon-stapled peptides as antibacterial agents
To address the challenge of antimicrobial resistance, we investigated new antibacterial peptides based on lysine- and leucine-rich sequences. We stabilised their membrane-active secondary structures by applying hydrocarbon stapling at sequence positions i and i+4. Stapling improved peptide structural stability in both aqueous and lipid environments, regardless of the staple position. It also enhanced antibacterial efficiency against both gram-negative and gram-positive bacteria, including antibiotic-resistant strains, with minimum inhibitory concentrations (MICs) of 2 to 4 μM (2.5 to 5.5 μg/mL). The stapled peptides showed increased resistance to enzymatic degradation, particularly with staples incorporated near the N-terminus, and were not haemolytic or cytotoxic at their MICs. Molecular dynamics simulations revealed how stapling aids in (i) stabilising the membrane-active secondary structure of amphipathic peptides and (ii) accelerating their membrane insertion. Our results provide insight into peptide design for antimicrobial use. We show that hydrocarbon stapling of lysine- and leucine-rich short sequences may offer a pathway towards more stable and effective antibacterial agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


