交叉组装双功能催化剂催化n -芳基咔唑的反对称氨基选择性溴化反应
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
不对称催化卤代官能化因同时引入碳点手性和卤素官能化而受到重视。轴向手性双芳烯具有广泛的应用前景;然而,通过不对称卤化合成它们是有限的。在这里,我们报道了通过反对称氨基选择溴化合成C-N轴手性n -芳基咔唑。该过程通过由手性磷酸和非手性路易斯碱组成的催化剂混合物来促进。所得到的溴咔唑含有易于修饰的卤素手柄,允许通过非对映选择性交叉偶联引入额外的手性轴。这些轴向手性化合物具有较高的荧光量子产率和满意的圆偏振发光性能。机理研究表明,手性磷酸盐和非手性吡啶催化剂通过C-H非经典氢键和C-X卤素键交叉组装,为底物的有效对映面识别创造了一个有限的微环境。本研究为合成平面手性芳烃模拟物在材料科学中的应用铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
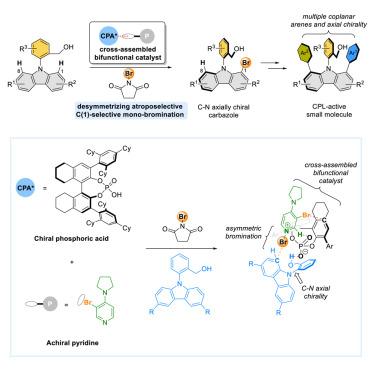

Desymmetrizing atroposelective bromination of N-arylcarbazoles enabled by cross-assembled bifunctional catalysts
Asymmetric catalytic halofunctionalization has gained prominence for introducing carbon point chirality and halogen functionality simultaneously. Axially chiral biaryls possess a range of promising applications; however, their synthesis through asymmetric halogenation is limited. Here, we report the synthesis of C–N axially chiral N-arylcarbazoles through desymmetrizing atroposelective bromination. This process is facilitated by a catalyst blend consisting of a chiral phosphoric acid and an achiral Lewis base. The resulting bromo-carbazoles contain readily modifiable halogen handles, allowing for the introduction of additional chiral axes through diastereoselective cross-coupling. These axially chiral compounds exhibit high fluorescence quantum yields and satisfactory circularly polarized luminescence performance. Mechanistic studies indicate that the chiral phosphate and achiral pyridine catalysts cross-assemble through C–H nonclassical hydrogen bonds and C–X halogen bonds, creating a confined microenvironment for efficient enantiofacial discrimination of substrates. This research paves the way for an approach to synthesizing mimics of planar chiral arenes for applications in materials science.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


