基于不同电解质溶液的 Zn 离子电容器
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
随着人们对碱离子电池中可燃性有机电解质安全风险的日益关注,以及对同一器件高能量密度和功率密度的追求,促使了对多价金属离子电容器的研究。锌离子电容器具有标准电位低、理论容量大、在水溶液中的安全性好等优点。我们证明了一种具有成本效益和高能量密度的锌离子基电容器,使用Zn作为负极,活性炭织物作为正极和Zn阳离子基1摩尔(M) ZnSO4, Zn(BF4)2, Zn(ClO4)2, Zn(TFSI)2或Zn(OTf)2水和非水电解质(乙腈(AN)和碳酸丙烯酯(PC))是可能的。计算出了基于Zn(ClO4)2和Zn(BF4)2的水基Zn离子电容器具有很高的能量和功率密度(在20kw kg - 1下为32wh kg - 1)。一些组装的ZICs(特别是基于Zn(BF4)2和Zn(OTf)2水溶液的ZICs)在10000次循环中表现出良好的循环和能量稳定性,而没有可测量的容量损失。计算了1 M Zn(BF4)2/AN基锌离子电容器的高能量密度(80 Wh kg−1)。经过3000次循环后,电池具有很好的电化学稳定性,表明Zn(TFSI)2/AN基ZIC电池具有较高的能效值(66.8%)。可测的能量效率依次递减:Zn(TFSI)2/AN >;锌(BF4) 2 / PC比;锌(TFSI) 2 / PC比;锌(传递)2 /一个比;锌(BF4) 2 /。本文章由计算机程序翻译,如有差异,请以英文原文为准。
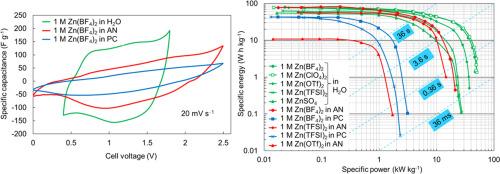
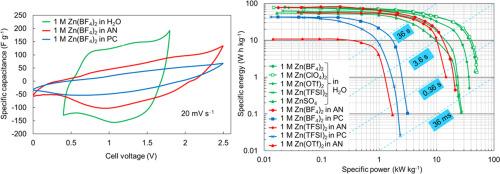
Zn-ion capacitors based on solutions of different electrolytes
The increasing concern on the safety risks associated with the flammable organic electrolytes in alkali-ion batteries and the pursuit of both high energy density and power density in one device has spurred the investigation of aqueous multivalent metal ion capacitors. Zinc-ion capacitors (ZICs) have the advantages of low standard potential, high theoretical capacity and good safety in aqueous electrolytes. We demonstrated that a cost effective and high energy density Zn-ion based capacitors, using Zn as the negative electrode, activated carbon fabric as the positive electrode and Zn cation based 1 molar (M) ZnSO4, Zn(BF4)2, Zn(ClO4)2, Zn(TFSI)2 or Zn(OTf)2 aqueous and non-aqueous electrolytes (acetonitrile (AN), and propylene carbonate (PC)) are possible. Very high energy and power densities (32 Wh kg−1 at 20 kW kg−1) have been calculated for aqueous Zn(ClO4)2 and Zn(BF4)2 based Zn-ion capacitors. Some assembled ZICs (in particular based on Zn(BF4)2 and Zn(OTf)2 aqueous electrolytes) had shown excellent cycling and energy stability over 10,000 cycles without measurable capacity loss. High energy densities (80 Wh kg−1) have been calculated for 1 M Zn(BF4)2/AN based Zn-ion capacitors. Very good electrochemical stability after 3000 cycles of cells has been achieved demonstrating reasonably high energy efficiency value (66.8 %) for Zn(TFSI)2/AN based ZIC cell. The energy efficiency measurable decreased in the order electrolytes: Zn(TFSI)2/AN > Zn(BF4)2/PC > Zn(TFSI)2/PC > Zn(OTf)2/AN > Zn(BF4)2/AN.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


