IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
YTHDF1作为一种关键的m6A读取蛋白,被认为是通过促进MHC-I降解而导致肿瘤细胞免疫逃避和抵抗的关键机制之一。我们正在探索将铁代谢调控与表观遗传调控相结合的治疗策略。在这里,我们开发了一种整合了地拉罗司(DFX,一种美国 FDA 批准的铁螯合剂)和 YTHDF1 siRNA(称为 PPD/siYTHDF1)的纳米组件,通过干扰铁代谢和敲除 YTHDF1 蛋白,共同促进肿瘤细胞的细胞周期停滞。同时,YTHDF1的缺乏会抑制溶酶体相关蛋白的mRNA翻译,上调MHC-I分子的表达(2.5倍),减少内化抗原的降解,增强T细胞介导的免疫反应,最终恢复肿瘤免疫监视,激发强大的抗肿瘤免疫功效。治疗后,肿瘤部位的 CD8+ T 细胞增加了 2.2 倍,脾脏中的效应记忆 T 细胞增加了 2.1 倍。它在乳腺癌治疗、术后抗复发和抗转移模型中均显示出高效的抗肿瘤效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。

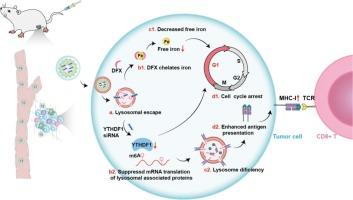
YTHDF1-targeting nanoassembly reverses tumoral immune evasion through epigenetics and cell cycle modulation
YTHDF1, as a key m6A reader protein, is believed to be one of the key mechanisms leading to tumor cell immune evasion and resistance via promoting MHC-I degradation. We explore therapeutic strategies that combine iron metabolism regulation with epigenetic regulation. Here, a nanoassembly that integrates Deferasirox (DFX, an FDA-approved iron chelator) and YTHDF1 siRNA (known as PPD/siYTHDF1) has been developed, which jointly promotes cell cycle arrest in tumor cells by interfering with iron metabolism and knocking down YTHDF1 protein. At the same time, YTHDF1 deficiency inhibits the mRNA translation of lysosome-related proteins, upregulates MHC-I molecule expression (2.5-fold), reduces the degradation of internalized antigens, enhances T cell-mediated immune response, and ultimately restores tumor immune surveillance and triggers powerful anti-tumor immune efficacy. After treatment, CD8+ T cells in the tumor site increased by 2.2-fold, and effector memory T cells in the spleen increased by 2.1-fold. It demonstrates a highly effective anti-tumor effect in breast cancer treatment, as well as in postoperative anti-recurrence and anti-metastasis models.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


