IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
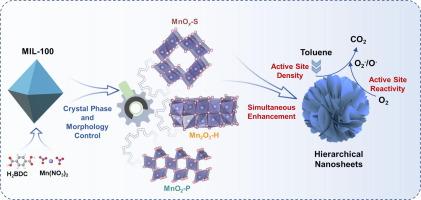
吸附和活化在异相反应中至关重要,尤其是在甲苯催化氧化中。如何协同增强这两个过程以提高甲苯氧化活性仍然是一个重大挑战。我们采用了一种简便的 MOFs 牺牲与碱性溶液后处理相结合的改性策略来制备分层结构的 MnOx 纳米片(MnOx-S)催化剂。与通过直接热解 Mn-MOFs 和 Mn(NO3)2 前驱体获得的 Mn2O3-H 和 MnO2-P 催化剂相比,MnOx-S 催化剂在甲苯氧化催化活性方面有明显改善。T90 分别降低了 26°C 和 64°C。究其原因,这与多孔纳米片状结构密切相关,这种结构具有高易接近表面和高密度暴露的活性位点,从而有利于反应分子的吸附/活化。同时,MnOx-S 催化剂的强氧化还原能力提高了氧的流动性和反应活性,使催化反应速率比 MnO2-P 提高了 12.4 倍。苯甲酸盐的积累和转化是甲苯氧化反应的限速步骤,该反应在三种不同的催化剂表面上进行。MnOx-S 催化剂的分层结构明显加快了这一关键步骤。这项工作推动了对 MOF 相关催化材料的研究,并提供了一种同时增强吸附和反应过程的双重方法,有助于设计用于降解挥发性有机化合物的高性能催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Facile preparation of MnOx catalysts derived from MOFs for efficient toluene Oxidation: Synergistic enhancement of active site density and reactivity
Adsorption and activation are essential in heterogeneous reactions, particularly for toluene catalytic oxidation. Synergistically enhancing both processes to boost toluene oxidation activity remains a significant challenge. A facile MOFs sacrificial combined with an alkaline solution post-treatment modification strategy was implemented to prepare hierarchically structured MnOx nanosheets (MnOx-S) catalysts. Compared with the Mn2O3-H and MnO2-P catalysts, obtained by the direct pyrolysis of Mn-MOFs and Mn(NO3)2 precursors, the MnOx-S catalyst exhibits a noticeable improvement in catalytic activity for toluene oxidation. The T90 was reduced 26 °C and 64 °C, respectively. The reason is closely related to the porous nanosheet structure, possessing a highly accessible surface and high density of exposed active sites, thus facilitating the adsorption/activation of reactant molecules. Meanwhile, the strong redox ability in the MnOx-S catalyst boosted oxygen mobility and reactivity, resulting in a 12.4-fold catalytic reaction rate compared with MnO2-P. The accumulation and conversion of benzoates is the rate-limiting step in the toluene oxidation reaction occurring on three distinct catalyst surfaces. This critical step is notably expedited by especially hierarchical structures in MnOx-S catalysts. This work advances the investigation of MOF-related catalytic materials and provides a dual approach that simultaneously enhances both adsorption and reaction processes, facilitating the design of high-performance catalysts for VOCs degradation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


