空心亚微球Ni/ co - promoting CaO/Ca12Al14O33吸附增强水气转换制氢及CaCO3原位CO2转化
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
CaO吸附剂/催化剂双功能材料在吸附增强型氢气生产(如吸附增强型水气转换)中具有广阔的应用前景。为了实现CO2就地捕集利用同时制氢,提出了吸附强化水气转换与CaCO3 CH4重整就地CO2转化的一体化工艺。这项工作的重点是定制设计一种CaO吸附剂/催化剂双功能材料,在这种集成过程中既能高效产氢,又能就地转化二氧化碳。首次提出了水热碳化后自还原和水蒸气气化模板去除的模板辅助策略,以获得空心亚微球Ni/ co -促进CaO/Ca12Al14O33。由于独特的空心亚微球结构和增强的催化活性,合成的材料在集成过程中表现出高而稳定的H2产氢、CO2捕获和原位CO2转化性能。Ni-Co相互作用促进了氧空位和Ni-Co合金,这是水气转换和CH4-CaCO3反应的活性催化位点。此外,还证实了氧空位介导CaCO3在空心亚微球Ni/共促进CaO/Ca12Al14O33上CH4重整的机理。经过20个循环后,合成材料的吸附增强水气转换CO转化率保持在97.0%,CH4转化率高达95.1%,CaCO3的CH4重整H2/CO摩尔比接近1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
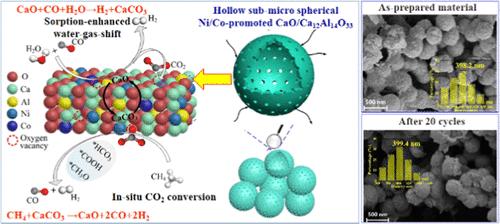
Hollow Submicrospherical Ni/Co-Promoted CaO/Ca12Al14O33 for H2 Production from Sorption-Enhanced Water–Gas Shift with In Situ CO2 Conversion via CH4 Reforming of CaCO3
CaO sorbent/catalyst bifunctional materials are promising for CO2 capture in sorption-enhanced H2 production such as sorption-enhanced water–gas shift. For simultaneous H2 production with CO2 in situ capture and utilization, the integrated process of sorption-enhanced water–gas shift and in situ CO2 conversion by CH4 reforming of CaCO3 was proposed. This work focused on the tailored design of a CaO sorbent/catalyst bifunctional material for both efficient H2 production and in situ CO2 conversion in this integrated process. The template-assisted strategy of hydrothermal carbonization followed by self-reduction and template removal via steam gasification was first proposed to obtain the hollow submicrospherical Ni/Co-promoted CaO/Ca12Al14O33. The as-synthesized material exhibits high and stable H2 production, CO2 capture, and in situ CO2 conversion performance in the integrated process due to the unique hollow submicrospherical structure and enhanced catalytic activity. Ni–Co interaction boosts oxygen vacancy and Ni–Co alloy, which are the active catalytic sites for the water–gas shift and CH4–CaCO3 reactions. Moreover, the oxygen vacancy-mediated mechanism on CH4 reforming of CaCO3 over the hollow submicrospherical Ni/Co-promoted CaO/Ca12Al14O33 is confirmed. After 20 cycles, CO conversion from sorption-enhanced water–gas shift using the as-synthesized material retains 97.0%, accompanied by high CH4 conversion of 95.1% and the H2/CO molar ratio close to unity from CH4 reforming of CaCO3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


