四氢喹啉与二硒化物†的可调选择性电化学硒化
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
提出了一种简单、环保的四氢喹啉与二硒化物硒化反应的电化学合成方法。通过调整反应条件,可以合成不同的产物。以NaI为电解质,2,2,6,6-四甲基哌啶氧基(TEMPO)为氧化还原介质,成功合成了C-3硒化喹啉,实现了具有挑战性的C-3取代喹啉。在没有TEMPO的情况下,得到了C-6硒化四氢喹啉。这种绿色的方法具有优异的选择性和广泛的底物范围,反应条件可以很容易地调整以获得不同的结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
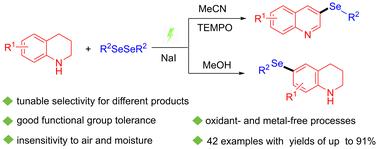
Tunable selective electrochemical selenization of tetrahydroquinolines with diselenides†
A simple and environmentally friendly electrochemical synthesis method is presented for the selenylation of tetrahydroquinolines with diselenides. By adjusting the reaction conditions, different products can be synthesized. Using NaI as the electrolyte and 2,2,6,6-tetramethylpiperidinooxy (TEMPO) as a redox mediator, C-3 selenylated quinolines were successfully synthesized, achieving the challenging C-3 substitution of quinolines. In the absence of TEMPO, C-6 selenylated tetrahydroquinolines were obtained. This green method offers excellent selectivity and a broad substrate scope, with reaction conditions that can be easily tuned to achieve different outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


