流感病毒诱导的I型干扰素破坏肺泡上皮修复和发育中的肺紧密连接的完整性。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
最近,我们证明了甲型流感病毒(IAV)感染的缺乏功能性IFN-I受体(IFNAR-/-)的小鼠新生儿与野生型(WT)新生儿相比,存活率显著提高,肺部病理减少。与之形成直接对比的是,成年IFNAR-/-小鼠在IAV感染后的发病率高于成年WT小鼠。我们假设IAV在初生新生儿II型肺泡上皮细胞(TIIECs)中诱导IFN-I信号传导,IAV是IAV感染的主要细胞类型,也是肺部宿主反应的发起者,促进了年龄特异性病毒发病。纯化TIIECs的多因素转录分析显示,年龄,而不是感染状态,是TIIECs转录差异的主要驱动因素。随后的通路分析表明,与WT新生儿相比,iav感染的IFNAR-/-新生儿在感染后2天(dpi)显著上调细胞增殖、组织修复和紧密连接基因。接下来,为了确定这些生长和修复差异是否在感染后期持续存在,在6 dpi时进行了修复基因表达的靶向分析和肺密封紧密连接分子ZO-1和occludin的免疫荧光定量。与WT新生儿相比,IFNAR-/-新生儿的全肺occludin染色和修复基因表达明显更高。总之,我们的数据表明,IFN-I信号通过破坏肺泡修复和肺屏障完整性,在发育中的肺中具有极高的致病性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
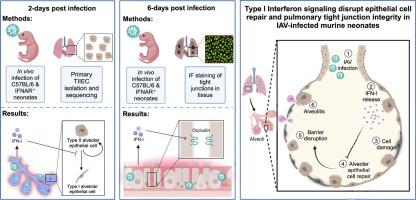
Influenza virus-induced type I interferons disrupt alveolar epithelial repair and tight junction integrity in the developing lung
Recently, we demonstrated that influenza A virus (IAV)-infected murine neonates lacking a functional IFN-I receptor (IFNAR−/−) had significantly improved survival and reduced lung pathology relative to wild-type (WT) neonates. In direct contrast, adult IFNAR−/− mice display enhanced morbidity following IAV infection relative to WT adults. We hypothesized that IAV-induced IFN-I signaling in primary neonatal type II alveolar epithelial cells (TIIECs), the main cell type of IAV infection and initiator of host response in the lung, contributed to age-specific viral pathogenesis. Multifactorial transcriptional analysis of purified TIIECs revealed age, not infection status, as the primary driver of transcriptional differences in TIIECs. Subsequent pathway analysis demonstrated IAV-infected IFNAR−/− neonates significantly upregulated cell proliferation, tissue repair and tight junction genes at 2-days post-infection (dpi), compared to WT neonates. Next, to determine if these growth and repair differences persisted later in infection, targeted analysis of repair gene expression and immunofluorescent quantification of pulmonary sealing tight junction molecules ZO-1 and occludin was performed at 6-dpi. Relative to WT neonates, IFNAR−/− neonates had significantly higher whole lung occludin staining and repair gene expression. Together, our data demonstrates IFN-I signaling is extremely pathogenic in the developing lung by disrupting alveolar repair and pulmonary barrier integrity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mucosal Immunology
医学-免疫学
CiteScore
16.60
自引率
3.80%
发文量
100
审稿时长
12 days
期刊介绍:
Mucosal Immunology, the official publication of the Society of Mucosal Immunology (SMI), serves as a forum for both basic and clinical scientists to discuss immunity and inflammation involving mucosal tissues. It covers gastrointestinal, pulmonary, nasopharyngeal, oral, ocular, and genitourinary immunology through original research articles, scholarly reviews, commentaries, editorials, and letters. The journal gives equal consideration to basic, translational, and clinical studies and also serves as a primary communication channel for the SMI governing board and its members, featuring society news, meeting announcements, policy discussions, and job/training opportunities advertisements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


