新型二酰胺共价有机聚合物 (COP),用于从高酸性核气流中捕获锕系元素 U(VI)、Pu(IV) 和 Am(III)
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。

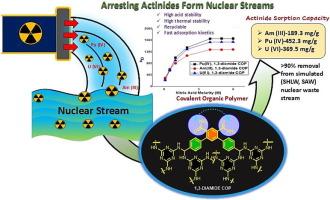
Novel diamide covalent organic polymers (COPs) for arresting actinides U(VI), Pu(IV) and Am(III) from highly acidic nuclear stream
Targeted towards the hitherto unaddressed challenge of actinide capture under harsh radiochemical conditions (nitric acid molarity ≥2 M, gamma radiation field 500 kGy), novel diamide covalent organic polymers (COPs) were successfully synthesized and characterized. The completely incinerable 1,3 and 1,4-Diamide COPs were endowed with exceptional radiochemical stability, high surface area (>1400 m2 g−1) and remarkable sorption capacity for the actinides viz. U(VI), Pu(IV) and Am(III). Batch sorption studies with Am(III), Pu(IV) and U(VI) from nitric acid medium revealed higher uptake with 1,3 over 1,4-Diamide COP, in general. Distribution co-efficient, kD was found to enhance with nitric acid molarity, attaining saturation after 4 M. Sorption was found to follow pseudo second order kinetics, Langmuir adsorption isotherm and was enthalpy driven. 1,3-Diamide COPs yielded high sorption capacity for U(VI), Pu(IV) and Am(III) (368.8, 452.3 and 189.3 mg g−1 respectively), from 4 M nitric acid medium. Actinide removal was >90 % from simulated nuclear streams. Mode of complexation of COPs with uranyl was probed experimentally by X-ray Photoelectron Spectroscopy and Fourier transform Infrared Spectroscopy. Additional insights for optimized ligand and metal–ligand binding geometries were obtained by theoretical modelling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


