IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
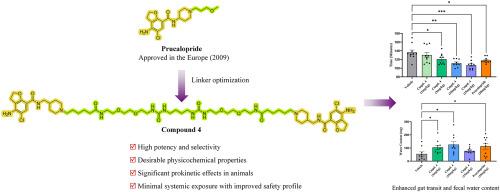
慢性特发性便秘(CIC)是一种普遍存在的胃肠道疾病,但能兼顾疗效和安全性的治疗方案却很有限。目前的疗法,如 5-HT4 受体(5-HT4R)激动剂普鲁卡必利,显示出疗效,但往往与全身副作用有关,这突出表明需要肠道限制性替代品。在此,我们首次报道了通过整合普鲁卡必利和替那潘诺的药理作用,合理设计和合成靶向粘膜 5-HT4R 的肠道限制性二价激动剂。通过结构优化,特别是链接长度和性质的优化,发现了化合物 4,该化合物具有强效的 5-HT4R 激动活性、高选择性和良好的理化性质。临床前研究表明,化合物 4 能显著增强整个肠道和结肠的转运,增加粪便排出量和水分含量,同时保持最小的全身吸收,证实了其肠道限制性。这些发现强调了肠道限制型 5-HT4R 激动剂作为 CIC 新型治疗策略的可行性,并为开发更安全、更有效的胃肠道疾病治疗方法提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Novel gut-restricted bivalent agonists targeting mucosal 5-HT4R: Design, synthesis, and biological evaluation
Chronic idiopathic constipation (CIC) is a prevalent gastrointestinal disorder with limited therapeutic options that balance efficacy and safety. Current therapies, such as the 5-HT4 receptor (5-HT4R) agonist prucalopride, demonstrate efficacy but are often associated with systemic side effects, highlighting the need for gut-restricted alternatives. Herein, we report for the first time the rational design and synthesis of gut-restricted bivalent agonists targeting mucosal 5-HT4R by integrating pharmacophores of prucalopride and tenapanor. Structural optimization, particularly of linker length and properties, led to the discovery of compound 4, which exhibited potent 5-HT4R agonistic activity, high selectivity, and favorable physicochemical properties. Preclinical studies demonstrated that compound 4 significantly enhanced whole-gut and colonic transit, increased fecal output and water content, while maintaining minimal systemic absorption, confirming its gut-restricted nature. These findings underscore the feasibility of gut-restricted 5-HT4R agonists as a novel therapeutic strategy for CIC and provide valuable insights into the development of safer, more effective treatments for gastrointestinal disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


