同时调节 PPARγ、COX-2 和 15-LOX 的新型噻唑啉酮类药物,用于治疗代谢性疾病相关的门静脉炎症
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。

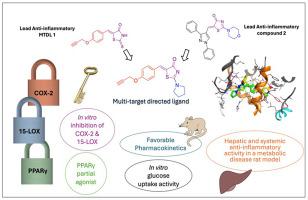
Novel thiazolones for the simultaneous modulation of PPARγ, COX-2 and 15-LOX to address metabolic disease-associated portal inflammation
A hybrid pharmacophore model, based on structural motifs previously identified by our team, was employed to generate ligands that simultaneously target COX-2, 15-LOX, and PPARγ in the context of metabolic dysfunction-associated fatty liver disease (MAFLD). Notable COX-2 inhibitory activities (IC50 = 0.065–0.24 μM) were observed relative to celecoxib (IC50 = 0.049 μM). The two most effective 15-LOX inhibitors, 2a and 2b, exhibited 69 % and 57 % of quercetin's action, respectively. Utilizing the rat hemi-diaphragm model to assess in vitro glucose uptake capacity, compounds 2a and 2b demonstrated significant glucose uptake potential in the absence of insulin, surpassing that of pioglitazone. Compound 2a activated PPARγ with an EC50 value of 3.4 μM in a Gal4-hybrid reporter gene assay, indicating partial agonistic action. Interesting binding interactions with targets of interest were identified by molecular docking studies. As well, the expression levels of 20-HETE, Il-1β and TNF-α were decreased in LPS-challenged RAW264.7 macrophages upon treatment with compound 2a. The pharmacokinetic analysis of 2a and assessment of its in vivo efficacy in addressing hepatic impairment in rat models of diabetes and pre-diabetes were carried out. Together, these findings may offer preliminary insights into the potential of these compounds for further refinement in the existing therapeutic arsenals for metabolic diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


