用 ClickZip 质量标签标记的肽的液相色谱-电感耦合等离子体质谱分析
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
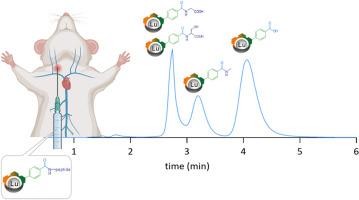
Liquid chromatography–inductively coupled plasma mass spectrometry analysis of peptides labelled with ClickZip mass tags
Lipidated anorexigenic peptides are highly promising compounds for the treatment of obesity and related diseases. However, their exact mechanism of action still remains unknown. We labelled a lipidated analogue of an anorexigenic prolactin-releasing peptide (palm11-PrRP31) with an extremely stable ClickZip lanthanide tag, facilitating tracking of the peptide within the organism. We then employed a separation method based on liquid chromatography combined with inductively coupled plasma mass spectrometry (LC–ICP-MS). This technique involved the use of an unconventional mobile phase containing 5% 1,2-hexanediol in H2O (v/v) with the addition of 2% formic acid. Using a rapid6-min analysis, we were able to quantify the ClickZip tag – and thus indirectly the fate of the labelled peptides in the living organism – independently of free Ln3+ ions. The detection limits for the various lanthanide chemical forms were extremely low, ranging between 0.9 and 3.4 ng/L. We demonstrated the suitability of the method for analysing real biological samples like blood plasma, and confirmed the accuracy of our results. Prior to LC–ICP-MS analysis, we optimised a process involving the microwave-assisted digestion of liver samples to preserve the integrity of the ClickZip tag. We also identified several metabolites of the labelled peptides in the liver, urine, and blood plasma, highlighting the utility of the method for revealing the mechanism of action behind the labelled lipopeptides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


