使用 Cu/MCM-41 对含左氧氟沙星废水进行机器学习催化湿过氧化反应
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
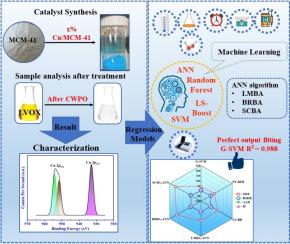
Machine learning enabled catalytic wet peroxidation of levofloxacin bearing wastewater using Cu/MCM-41
Levofloxacin (LVOX) is a widely used antibiotic and persistent in environment that causes major health and environmental risks. The present study investigated the catalytic wet peroxidation (CWPO) of LVOX wastewater using different weight percentages of copper (0.5–5 wt%) on MCM-41. Among these, 1 % Cu/MCM-41 showed better catalytic activity for the remove of LVOX. The surface area and pore volume of the MCM-41 decreased upon copper loading onto the MCM-41 framework (Cu/MCM-41), from 712 m2/g to 605 m2/g and 0.987 mL/g to 0.816 mL/g, respectively. X-ray photoelectron spectroscopy (XPS) analysis confirmed the presence of Si 2p, O 1 s, and C 1 s at consistent binding energies across all samples. However, in Cu/MCM-41, an additional Cu 2p peak was detected at 933.3 eV, that indicates the successful incorporation of copper species on MCM-41 framework. The maximum LVOX removal was observed 94 % and mineralization was observed 64 % through CWPO process at optimized reaction conditions of pH 10, a catalyst dosage of 1 g/L, H2O2 of 13.7 mmol/L, LVOX initial concentration of 500 mg/L, temperature of 333 K, and residence time of 180 min. LVOX mineralization kinetics follows a pseudo-first-order reaction with an R2 of 0.99. The thermodynamic study revealed that the CWPO of LVOX is non-spontaneous and endothermic in nature. Machine learning (ML) models were deployed to analyze the experimental data, including Gaussian support vector machine (G-SVM), Fine Tree-Random Forest regression (FT RFR), LS-boost regression (LS-BR), and Artificial neural network (ANN). The G-SVM, FT-RFR, LS-BR, and ANN model showed an adequate prediction of the response, with absolute average deviation (AAD) of 0.462, 1.71, 2.401, and 2.917 and root mean squared error (RMSE) of 6.531, 7.700, 9.346 and 9.665, respectively. Among these models, G-SVM demonstrated the highest prediction accuracy, with the lowest RMSE and AAD compared to other fitted models.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


