芳香族氨基甲酸酯农药的直接光降解:水介质与非水介质中的动力学和机理
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
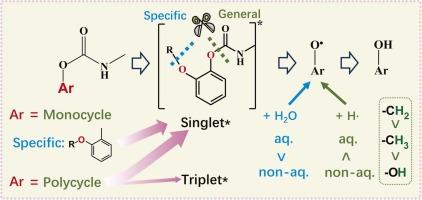
Direct photodegradation of aromatic carbamate pesticides: Kinetics and mechanisms in aqueous vs. non-aqueous media
The direct photodegradation quantum yields (Φ) of five representative aromatic carbamate pesticides - carbaryl, carbofuran, propoxur, isoprocarb, and metolcarb - were examined in both aqueous and non-aqueous solutions, the latter mimicking hydrophobic environments such as leaf surfaces. For carbaryl, carbofuran, isoprocarb, and metolcarb, the Φ values generally followed the order Φwater < ΦMeOH < Φn-hexane, while propoxur showed a different trend, ΦMeOH < Φn-hexane < Φwater. Scavenging and laser flash photolysis experiments, combined with quantum chemical calculations, were used to clarify the photodegradation mechanisms. Photodegradation is primarily initiated by the singlet excited state (S*), with the triplet state (T*) also contributing in compounds with conjugated structures, such as carbaryl. Upon excitation, MCAEs generated both radical cations (S•+) and phenoxyl radicals (S-O•), and S•+ would convert to S-O• subsequently. S-O• is predominantly generated through the cleavage of C-O bonds in ester groups, subsequently abstracting hydrogen from solvent molecules. The reactivity of hydrogen donors in these solvents follows the order: -CH2- > -CH3 > -OH. For propoxur, the ether group also contributes to the formation of S-O•, which further reacts with H2O and enhances degradation in aqueous environments. Solvent polarity had a minimal effect on photodegradation. This comparative study of degradation in aqueous and nonaqueous phases provides insights for designing and selecting pesticides that are effective during use in nonaqueous environments, such as on leaf surfaces, yet degrade rapidly in aqueous environments in the post-application phase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


