基于Lewis酸改性剂的燃料电池耐h2s氢氧化电催化剂
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
工业氢燃料通常含有约 5 ppm 的硫化氢 (H2S),会对燃料电池中的碳化铂 (Pt/C) 催化剂造成不可逆的毒害。在实际使用中,H2S 的去除率应低于 4 ppb;但这一过程具有挑战性,而且成本高昂。我们介绍了一种耐 H2S 但性能优异的氢氧化反应(HOR)催化剂,其制备方法是将氧化铬(Cr2O3)化学接枝到钼-镍(MoNi4)合金上。Cr2O3 作为一种路易斯酸,可增强对羟基离子的特异性吸附,从而通过静电排斥阻止 S2- 扩散到催化剂表面。同时,吸附的羟基物种通过改善电双层中的氢键网络,促进了 HOR 动力学。复合催化剂在碱性电解质中的氢氧还原性能可与商用 Pt/C 相媲美。此外,使用这种催化剂作为阳极的燃料电池可在 5 ppm 的 H2S 中存活而不会失活,而 Pt/C 催化剂则会迅速降解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
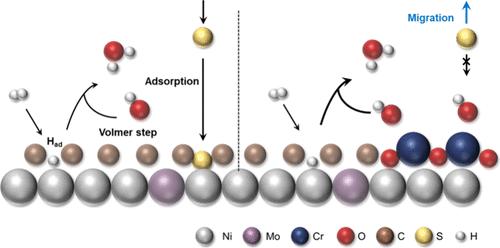
An Efficient H2S-Tolerant Hydrogen Oxidation Electrocatalyst Enabled by a Lewis Acid Modifier for Fuel Cells
Industrial hydrogen fuel typically comprises about 5 ppm of hydrogen sulfide (H2S), incurring irreversible poisoning of platinum on carbon (Pt/C) catalyst in fuel cells. For realistic use, H2S should be removed to below 4 ppb; this process, however, is challenging and costly. We describe an exceptional H2S-tolerant yet high-performing hydrogen oxidation reaction (HOR) catalyst prepared by chemical grafting of chromic oxide (Cr2O3) onto a molybdenum–nickel (MoNi4) alloy. Cr2O3 as a Lewis acid enhances the specific adsorption of hydroxyl ions, which in turn prevents from S2– diffusing to the catalyst surface via electrostatic repulsion. Meanwhile, the adsorbed hydroxyl species boost HOR kinetics through improving the hydrogen-bond networks in electrical double layers. The composite catalyst achieved HOR performance comparable to that of commercial Pt/C in an alkaline electrolyte. Moreover, a fuel cell using this catalyst as anode can survive 5 ppm of H2S without deactivation, compared with rapid degradation observed over the Pt/C counterpart.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


