cu /BiVO4的硫缺陷调谐及氢自由基在光电化学制氨中的作用
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在常温和中性条件下,光电化学硝酸还原是一种很有前途的氨(NH3)生产方法。而在硝酸还原过程中,加氢是NH3选择性生成的关键过程;因此,诱导活性氢用于硝酸加氢并抑制产氢是一个值得关注的问题。本研究构建了BiVO4/ cu (BVO/CS)异质结构用于电化学硝酸还原反应(PEC NIRR)。cu的引入优化了BVO/CS的电子传递能力,提高了BVO/CS的表面催化动力学。同时,表面硫空位的存在促进了硝酸盐的吸附和活化,实现了H2O的分裂,成功生成了丰富的氢自由基(H*)。生成的H*有效地用于NIRR的加氢。最佳BVO/CS的NH3产率和选择性分别达到30.55 μg h-1 cm-2和43.8%,分别是裸BVO的2.65倍和2.39倍。因此,这项工作确定了H*在硝酸盐加氢中的关键作用,为提高PEC NIRR提供了一种新的策略。成功制备了cu /BiVO4光电化学还原硝酸盐。硫缺陷使氢自由基生成,有效促进硝酸盐加氢和NH3的生成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
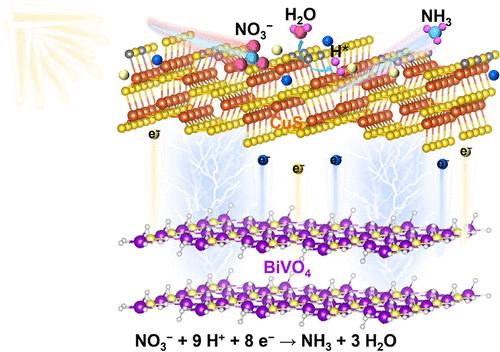
Sulfur Defect Tuning of CuS/BiVO4 and Understanding the Function of Hydrogen Radicals for Photoelectrochemical Ammonia Production
Photoelectrochemical nitrate reduction has been a promising method for ammonia (NH3) production under normal temperatures and neutral conditions. However, hydrogenation is a key process in the selective production of NH3 during nitrate reduction; therefore, inducing the active hydrogen for nitrate hydrogenation and inhibiting hydrogen production are a noteworthy problem. In this study, BiVO4/CuS (BVO/CS) heterostructure has been constructed for a photoelectrochemical nitrate reduction reaction (PEC NIRR). The introduction of CuS optimizes the electron-transfer ability and enhances the surface catalytic kinetics of BVO/CS. At the same time, the presence of sulfur vacancies on the surface promotes the adsorption and activation of nitrate, realizes the splitting of H2O, and successfully generates abundant hydrogen radicals (H*). The generated H* is effectively utilized in the hydrogenation of NIRR. The NH3 yield and selectivity of optimal BVO/CS reach 30.55 μg h–1 cm–2 and 43.8%, respectively, which are 2.65 and 2.39 times that of bare BVO. Therefore, this work determines the key role of H* for nitrate hydrogenation, providing a novel strategy for boosting PEC NIRR. CuS/BiVO4 was successfully fabricated for photoelectrochemical nitrate reduction. Sulfur defects enabled the generation of hydrogen radicals, which effectively promoted nitrate hydrogenation and NH3 production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


