双铜/光氧化还原催化自由基介导的亚胺酰化和烷基化反应
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
基于自由基的途径为从各种底物构建C(sp2/sp3) -S键提供了一种有吸引力的方法。本文报道了两种可用于芳基磺酰化的策略,以及芳基自由基介导的烷基碘化物与亚砜酰胺之间的交叉偶联反应,它们都是通过协同光氧化还原和铜催化进行的。这些温和、操作简单的反应具有广泛的底物范围和天然产物和药物分子后期功能化的潜在用途。此外,两种磺化反应都可以在连续流动条件下以克为单位进行。机理研究表明,通过空间位阻、富电子芳基自由基从碘化烷基中快速提取碘原子,以及通过改变配体来调整铜催化剂的电子性质,有助于烷基化的化学选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
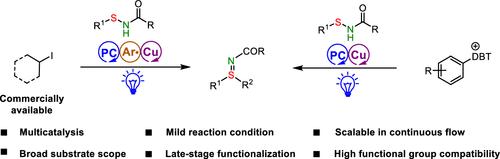
Dual Copper/Photoredox Catalysis for Radical-Mediated Arylation and Alkylation of Sulfenamides
Radical-based pathways provide an attractive approach for constructing C(sp2/sp3)–S bonds from various substrates. Herein we report two strategies that can be used for aryl radical sulfilimination of aryl sulfonium salts and aryl radical–mediated cross-coupling reactions between alkyl iodides and sulfenamides, both via synergetic photoredox and copper catalysis. These mild, operationally simple reactions have a broad substrate scope and potential utility for late-stage functionalization of natural products and drug molecules. In addition, both sulfilimination reactions can be carried out on a gram scale under continuous-flow conditions. Mechanistic studies indicate that rapid abstraction of the iodine atom from the alkyl iodide by sterically hindered, electron-rich aryl radicals and tuning of the electronic properties of the copper catalyst by varying the ligand contribute to the chemoselectivity for alkylation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


