STIM1 介导的 NFAT 信号与 STAT1 协同控制 T-bet 的表达和 TH1 的分化
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
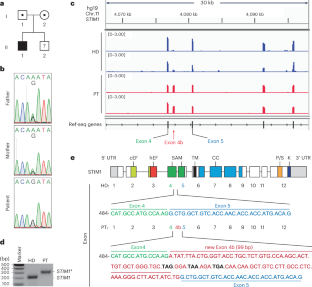
基质相互作用分子1 (STIM1)是存储操作Ca2+进入(SOCE)和T细胞活化的关键。T辅助性1 (TH1)细胞表达T-bet(由TBX21编码),介导对细胞内病原体的免疫。虽然已知SOCE调节其他TH谱系,但其在Th1分化中的作用尚不清楚。在这里,我们报告了一个STIM1中内含子功能丧失突变的患者,该突变消除了SOCE并导致免疫缺陷。我们证明SOCE促进活化T细胞核因子(NFAT)结合TBX21增强子中的保守非编码序列(CNS)-12,并使NFAT与STAT1协同介导TBX21的表达。虽然sce缺陷的CD4+ T细胞在缺乏白细胞介素-12 (IL-12)的情况下TBX21的表达降低,但它们的IL-12受体β1和β2的表达增加,使它们对IL-12信号通路敏感,并允许IL-12挽救T-bet的表达。我们的研究表明,stim1 - ssoe - nfat信号轴对Th1细胞的分化至关重要,这取决于细胞因子环境。本文章由计算机程序翻译,如有差异,请以英文原文为准。

STIM1-mediated NFAT signaling synergizes with STAT1 to control T-bet expression and TH1 differentiation
Stromal interaction molecule 1 (STIM1) is critical for store-operated Ca2+ entry (SOCE) and T cell activation. T helper 1 (TH1) cells, which express T-bet (encoded by TBX21), mediate immunity to intracellular pathogens. Although SOCE is known to regulate other TH lineages, its role in Th1 differentiation remains unclear. Here, we report a patient with an intronic loss-of-function mutation in STIM1, which abolishes SOCE and causes immunodeficiency. We demonstrate that SOCE promotes nuclear factor of activated T cells (NFAT) binding to conserved noncoding sequence (CNS)-12 in the TBX21 enhancer and enables NFAT to synergize with STAT1 to mediate TBX21 expression. While SOCE-deficient CD4+ T cells have reduced expression of TBX21 in the absence of interleukin-12 (IL-12), their expression of IL-12 receptors β1 and β2 is increased, sensitizing them to IL-12 signaling and allowing IL-12 to rescue T-bet expression. Our study reveals that the STIM1-SOCE–NFAT signaling axis is essential for the differentiation of Th1 cells depending on the cytokine milieu. Feske and colleagues show how STIM- and ORAI-dependent calcium activation of NFAT plus IFN–STAT1 signaling activates T-bet expression independently of IL-12 stimulation. This NFAT–STAT1 activation pathway is required for TH1 cell differentiation and protection against viral infections when IL-12 is missing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


