揭示掺杂 O 和 S 在改善 FeN4C 电化学氧气还原反应性能中的共性
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
杂原子掺杂的Fe-N-C催化剂由于其较低的成本而成为氧还原反应(ORR)中贵金属的有希望的替代品。然而,其增强性能的潜在机制,特别是电化学稳定性,仍然是一个有争议的话题。本研究利用密度泛函理论计算,结合恒定电位和隐式溶剂模型,研究了吡啶(PD-)和吡啶(PL-FeN4C)催化剂的电化学稳定性和活性。我们的研究结果表明配位氮原子的加氢敏感性是FeN4C催化剂中电化学稳定性的关键决定因素。此外,我们发现氧和硫掺杂对PD-FeN4C催化剂的整体ORR性能有相似的增强作用:(1)通过降低配位氮的p带中心,从而提高其抗氢化能力;(2)通过增加铁的价电子,从而增强对反应中间体的吸附,从而提高ORR活性。最后,我们的预测表明,O/ s掺杂的PL-FeN4C催化剂在酸性和碱性环境下都能显著提高电化学稳定性和卓越的ORR性能。这些见解有助于更深入地了解单原子催化剂(SACs)的微环境工程,并为开发前所未有的M-N-C催化剂提供有价值的指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
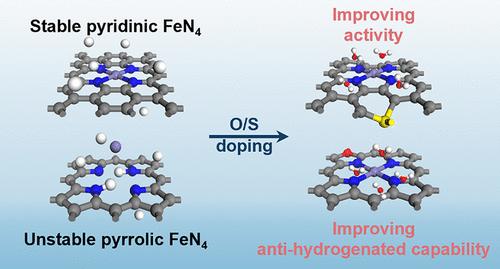
Unraveling the Common Nature of O and S Doping in Improving Electrochemical O2 Reduction Reaction Performance of FeN4C
Heteroatom-doped Fe-N-C catalysts have emerged as promising alternatives to noble metals for the oxygen reduction reaction (ORR) due to their lower cost. However, the underlying mechanisms responsible for their enhanced performance, particularly electrochemical stability, remain a subject of debate. This study leverages density functional theory calculations coupled with a constant potential and implicit solvent model to investigate the electrochemical stabilities and activities of pyridinic (PD-) and pyrrolic FeN4C (PL-FeN4C) catalysts. Our findings reveal that the hydrogenation susceptibility of coordinating nitrogen atoms is a critical determinant of electrochemical stability within FeN4C catalysts. Moreover, we demonstrate that oxygen and sulfur doping exerts similar effects on enhancing the overall ORR performance of PD-FeN4C catalysts: (1) by reducing the p-band center of the coordinating nitrogen, thereby improving their resistance to hydrogenation, and (2) by increasing the valence electrons of iron, leading to stronger adsorption of reaction intermediates and consequently enhanced ORR activity. Finally, our predictions suggest that O/S-doped PL-FeN4C catalysts could achieve significantly improved electrochemical stability and superior ORR performance in both acidic and alkaline environments. These insights contribute to a deeper understanding of microenvironment engineering in single-atom catalysts (SACs) and offer valuable guidelines for the development of unprecedented M-N-C catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


