姜黄素光稳定Mn(II)配合物用于有效的光动力治疗和精确的体内三维肿瘤成像
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
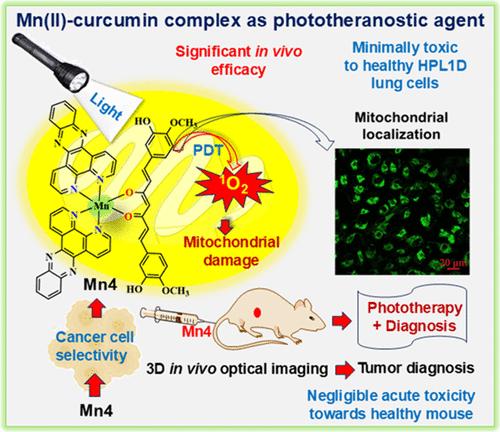
具有发射特性的第一行过渡金属光活性配合物为癌症治疗提供了双重途径,实现了精确的光学肿瘤检测和随后的用光根除。我们报道了一种具有光稳定性和光活性的混合配体Mn(II)配合物,Mn4,具有天然存在的姜黄素配体和双吡啶吩嗪碱。Mn4在体内表现出明显的可见光和红光引发的对癌细胞的光毒性和精确的肿瘤成像能力。该配合物在可见光区有一个吸收带,其尾部延伸到红色区,并在溶液中表现出良好的暗稳定性和光稳定性。Mn4在低能可见光(400-700 nm)和红光(660 nm)下对HeLa(宫颈癌)、A549(肺癌)和MCF-7(乳腺癌)细胞(IC50≈1.0 μM)以及三维多细胞肿瘤球体具有显著的光毒性。这种作用是由细胞毒性单线态氧介导的,并通过凋亡机制进行。重要的是,在相似的条件下,Mn4对正常HPL1D肺细胞和HEK-293肾细胞的毒性明显较低。细胞摄取研究显示,Mn4在A549癌细胞中选择性积累,具有线粒体定位,而在BEAS-2B正常肺细胞中的积累可以忽略不计。此外,三维光学肿瘤成像证实了Mn4在4T1乳腺荷瘤小鼠体内模型中的选择性肿瘤积累。采用4T1荷瘤原位小鼠模型的体内疗效研究表明,在低能量蓝色激光(450 nm)照射下,Mn4以剂量依赖的方式显著减少肿瘤体积和重量,突出了其作为有效光动力治疗(PDT)药物的潜力。毒理学研究证实,Mn4不会引起健康小鼠的生化或血液学参数异常。据我们所知,这是Mn(II)配合物与姜黄素的首次报道,也是金属配合物与姜黄素联合用于体内PDT和非侵入性3D光学肿瘤成像的第一个例子,为非大环Mn基癌症光疗铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Photostable Mn(II) Complex of Curcumin for Effective Photodynamic Therapy and Precise Three-Dimensional In Vivo Tumor Imaging
Photoactive complexes of first-row transition metals with emission properties offer a dual approach to cancer treatment, enabling precise optical tumor detection and subsequent eradication using light. We report a photostable and photoactive mixed-ligand Mn(II) complex, Mn4, featuring a naturally occurring curcumin ligand and dipyridophenazine base. Mn4 demonstrates significant visible and red light-triggered phototoxicity against cancer cells and precise tumor imaging capability in vivo. The complex exhibits an absorption band in the visible region, extending its tail into the red region, and shows excellent dark and photostability in solution. Mn4 induces significant phototoxicity against HeLa (cervical), A549 (lung), and MCF-7 (breast) cancer cells (IC50 ≈ 1.0 μM), as well as 3D multicellular tumor spheroids, under low-energy visible (400–700 nm) and red-light (660 nm). This effect is mediated by cytotoxic singlet oxygen and proceeds via an apoptotic mechanism. Importantly, Mn4 displays significantly lower toxicity toward normal HPL1D lung and HEK-293 kidney cells under similar conditions. Cellular uptake studies reveal selective accumulation of Mn4 in A549 cancer cells, with mitochondrial localization, and negligible accumulation in BEAS-2B normal lung cells. Furthermore, 3D optical tumor imaging demonstrated Mn4’s selective tumor accumulation in a 4T1 breast tumor-bearing in vivo mouse model. In vivo efficacy studies using a 4T1 tumor-bearing orthotopic mouse model show that Mn4 significantly reduces tumor volume and weight in a dose-dependent manner under low-energy blue laser (450 nm) irradiation, highlighting its potential as an effective photodynamic therapy (PDT) agent. Toxicological studies confirm that Mn4 does not induce abnormal biochemical or hematological parameters in healthy mice. To our knowledge, this is the first report of a Mn(II) complex with curcumin and the first example of a metal complex with curcumin for combined in vivo PDT and noninvasive 3D optical tumor imaging, paving the way for nonmacrocyclic Mn-based cancer phototheranostics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


