Bi2S3/Bi异质结光催化还原吸附去除Cr(VI
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
工业废水中铬(VI)具有高毒性和高迁移率,很难去除。本文采用真空合成的方法,将氧化铋(Bi2O3)和硫化铋(Bi2S3)在高温下反应生成单质铋,制备了Bi2S3/Bi异质结光催化剂。为了阐明光催化增强的机理,分析了Bi2S3和Bi2S3/Bi复合材料在可见光下光催化还原Cr(VI)的情况。与单一Bi2S3相比,光照30 min后,Bi2S3/Bi对Cr(VI)的还原率明显提高,表明其对Cr(III)的吸附能力较强。重复使用5次后,仍能有效去除Cr(VI)。Bi2S3/Bi光催化性能的提高在很大程度上是由于Bi元素在Bi2S3中形成了新的内置电场,促进了电子和空穴的分离和输运。此外,铋产生的SPR改善了光在Bi2S3表面的吸收和反射,促进了电子的转移。这一结论可以通过光电性能和仿真结果得到验证。综上所述,本研究为光催化剂的合成和废水中Cr(VI)的去除提供了重要的参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
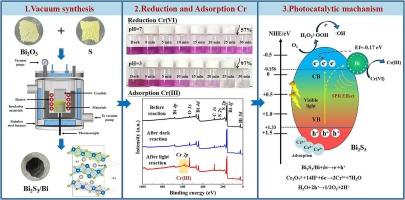
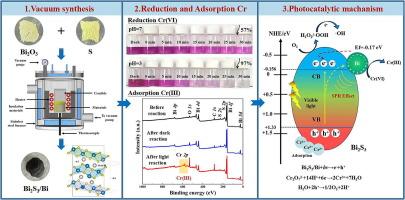
Bi2S3/Bi heterojunction for efficient removal of Cr(VI) via photocatalytic reduction and adsorption
It is difficult to remove Cr(VI) from industrial wastewater due to its high toxicity and high mobility. Herein, Bi2S3/Bi heterojunction photocatalysts were prepared through vacuum synthesis, in which bismuth oxide (Bi2O3) and bismuth sulfide (Bi2S3) reacted at high temperatures to generate elemental Bi. To elucidate the mechanism of photocatalytic enhancement, the photocatalytic reduction of Cr(VI) under visible light was analyzed for comparison between Bi2S3 and Bi2S3/Bi composites. Compared to single Bi2S3, Bi2S3/Bi performed much better in the reduction rate of Cr(VI) after 30 min of light irradiation, demonstrating its strong adsorption capacity for Cr(III). Moreover, Cr(VI) was still effectively removed after 5 times of reuse. To a large extent, the improvement in photocatalytic performance of Bi2S3/Bi is attributed to element Bi forming a new built-in electric field in Bi2S3, which facilitates the separation and transport of electrons and holes. In addition, the SPR generated by Bi improves the absorption and reflection of light on the Bi2S3 surface, promoting the transfer of electrons. This is a conclusion that can be validated by the photoelectric performance and simulation results. In summary, this study provides crucial reference for the synthesis of photocatalysts and the removal of Cr(VI) from wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


