探索电催化剂在碱性和酸性介质中析氧的综合比较综述
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
水被电化学分解,以一种环保的方式产生氢。两个半反应参与了水的电解过程,其中析氧反应(OER)是损失能量最大的反应。水电解在阳极过程中受到不良反应动力学的严重影响,使商业应用具有挑战性。与酸性方法相比,在碱性电解的特定要求下保持耐用的材料范围要广得多。因此,寻找在酸性介质中具有活性、可靠性和经济性的OER催化剂至关重要。基于已报道的电流密度为10 mA cm−2 (η10)时的过电位,本评估系统地检查了几种材料类别。收集了大量的研究,并概述了迄今为止文献中记录的OER催化剂。从这个角度来看,了解反应机理和利用先进的阳极催化剂都是必不可少的,前者为材料的结构工程提供了见解,以提高催化活性。然后对最近报道的几种酸性和碱性OER电催化剂进行了关键评价。最后,对今后OER催化剂的研究提出了几点建议。本文章由计算机程序翻译,如有差异,请以英文原文为准。
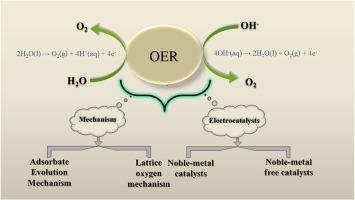
Exploring electrocatalysts for oxygen evolution: A comprehensive comparative review in alkaline and acidic medium
Water is electrochemically divided to produce hydrogen in an environmentally friendly manner. Two half-reactions contribute to the process of electrolyzing water, with the oxygen evolution reaction (OER) being the one that loses the most energy. Water electrolysis suffers significantly from poor reaction kinetics in its anodic process, making commercial use challenging. Compared to an acidic approach, the range of materials that remain durable under the specific requirements of alkaline water electrolysis is substantially broader. Therefore, identifying OER catalysts that are active, reliable, and economical in acidic media is crucial. Based on the reported overpotential at a current density of 10 mA cm−2 (η10), this evaluation systematically examines several material classes. A large number of studies are collected, and the OER catalysts documented in the literature to date are outlined. From this perspective, understanding the reaction mechanism and utilizing advanced anode catalysts are both essential, with the former providing insights for structural engineering of materials to enhance catalytic activity. A critical evaluation is then conducted on several recently reported acidic and basic OER electrocatalysts. Finally, a few recommendations for future research into OER catalysts are proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


