以124-恶二唑为基础:一系列不敏感含能材料的设计与合成及重排合成DNAF的新途径的发现
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
含有二硝基甲基的化合物由于其高密度和良好的氧平衡而成为含能材料的理想结构单元。然而,这些化合物通常有缺点,如分解温度低和机械灵敏度高,这限制了它们在含能材料中的实际应用。为了解决这些问题,我们成功地设计了一系列新的富含氮和氧的含能化合物7-11,这些化合物通过加入氨基来增强氢键。其中化合物8表现出优异的综合性能(Tdec = 215℃,ρ = 1.81 g cm-3, D = 8603 m s-1, IS >;40 J, FS >;360 N),表现出良好的典型二次爆炸特性。在合成过程中,开发了一种安全的合成3,4-二(硝胺)呋喃赞及其离子盐的新方法。通过多次实验探讨了1,2,4-恶二唑通过重排转化为1,2,5-恶二唑的机理。这种新的转变是对博尔顿-卡特里茨基型的唑-唑重排的有价值的补充。本文章由计算机程序翻译,如有差异,请以英文原文为准。
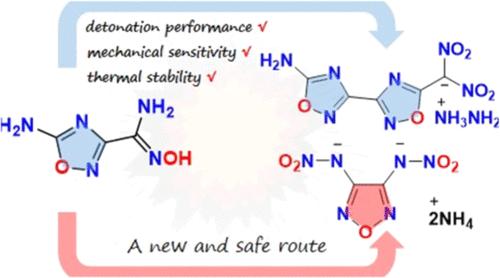
Based on 124-Oxadiazole: Design and Synthesis of a Series of Insensitive Energetic Materials and Discovery of Another Route for the Synthesis of DNAF via Rearrangement
Compounds containing dinitromethyl groups are ideal structural units for energetic materials due to their high density and good oxygen balance. However, these compounds often have drawbacks, such as low decomposition temperatures and high mechanical sensitivity, which limit their practical applications in energetic materials. In order to address these issues, a series of novel nitrogen- and oxygen-rich energetic compounds 7–11 have been successfully designed by incorporating amino groups to enhance hydrogen bonding. Among them, compound 8 exhibited an excellent overall performance (Tdec = 215 °C, ρ = 1.81 g cm–3, D = 8603 m s–1, IS > 40 J, FS > 360 N) and displayed good typical secondary explosive characteristics. During the synthesis process, a new and safe method was developed to synthesize 3,4-di(nitramino)furazan and its ion salts. The conversion from 1,2,4-oxadiazole to 1,2,5-oxadiazole via rearrangement was explored through multiple experiments to investigate its mechanism. This new transformation is a valuable complement to the azole–azole rearrangement of the Boulton–Katritzky type.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


