减数分裂DNA双链断裂形成的体外重建
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
Spo11复合体催化DNA双链断裂(DSBs)的形成,启动减数分裂重组——一个对生育和遗传多样性至关重要的过程1,2。虽然Spo11的功能已经被发现了27年,但之前在体外重建DSB形成的努力一直没有成功。在这里,我们对小鼠SPO11-TOP6BL蛋白复合物进行了生化表征,并表明该复合物在体外切割DNA并共价附着在DNA断裂的5 '端。使用点突变策略,我们发现Mg2+对体外该复合物的dna切割活性至关重要,通过敲入小鼠携带SPO11点突变证实,该突变破坏了其与Mg2+的结合,从而消除了DSB的形成。然而,SPO11复合物的活性与atp无关。我们还提供证据表明,小鼠SPO11复合物在生化上与祖先的拓扑异构酶VI不同。我们的发现为理解减数分裂重组的第一步建立了机制框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
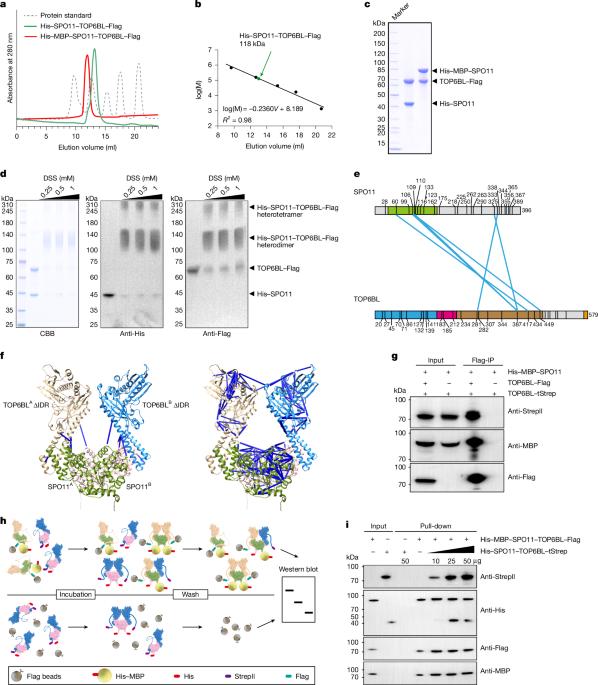
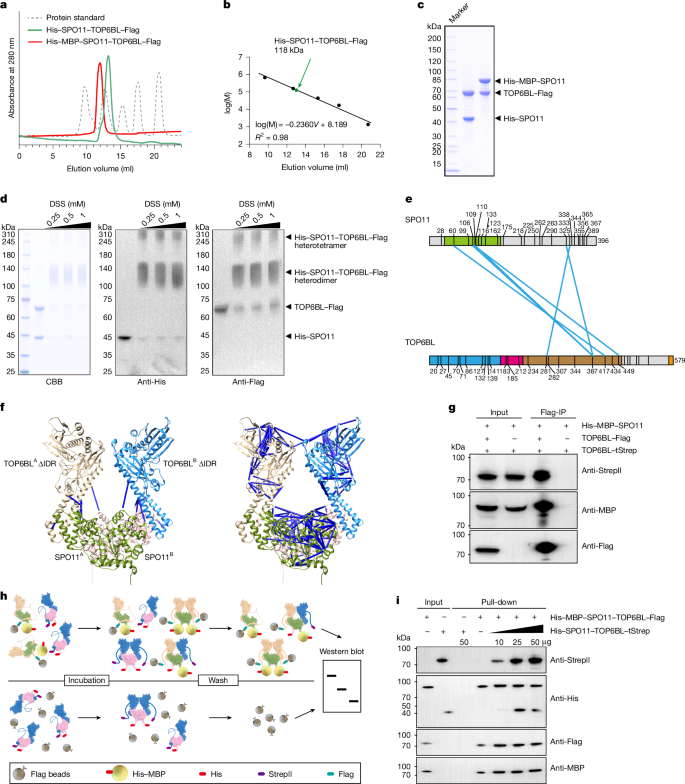
In vitro reconstitution of meiotic DNA double-strand-break formation
The Spo11 complex catalyses the formation of DNA double-strand breaks (DSBs), initiating meiotic recombination—a process that is essential for fertility and genetic diversity1,2. Although the function of Spo11 has been known for 27 years, previous efforts to reconstitute DSB formation in vitro have been unsuccessful. Here we biochemically characterize the mouse SPO11–TOP6BL protein complex, and show that this complex cleaves DNA and covalently attaches to the 5′ terminus of DNA breaks in vitro. Using a point-mutation strategy, we reveal that Mg2+ is essential for the DNA-cleavage activity of this complex in vitro, as confirmed by knock-in mice carrying a point mutation in SPO11 that disrupts its binding to Mg2+, thereby abolishing DSB formation. However, the activity of the SPO11 complex is ATP-independent. We also present evidence that the mouse SPO11 complex is biochemically distinct from the ancestral topoisomerase VI. Our findings establish a mechanistic framework for understanding the first steps of meiotic recombination. The in vitro characterization of a key event in the early stages of meiosis—the induction of double-strand DNA breaks by the SPO11–TOP6BL complex—provides insight into the catalytic mechanism and evolution of SPO11.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


