弥漫性中线胶质瘤中gaba能神经元到胶质瘤突触
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
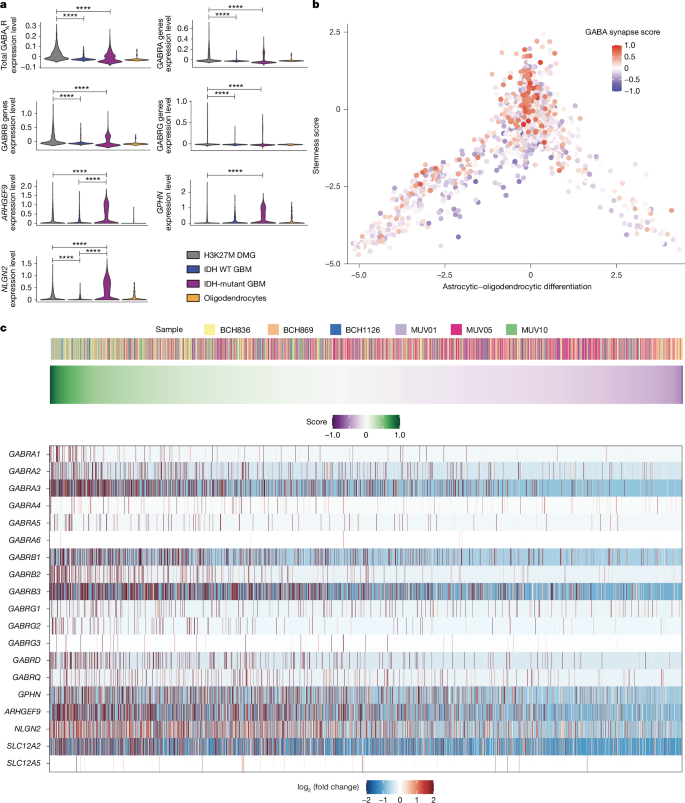
高级别胶质瘤(HGGs)是脑癌相关死亡的主要原因。HGGs包括临床、解剖和分子上不同的亚型,可分层为弥漫性中线胶质瘤(dmg),如h3k27m改变的弥漫性固有脑桥胶质瘤,以及半球HGGs,如IDH野生型胶质母细胞瘤。神经元活动通过旁分泌信号传导1、2和神经元-胶质瘤突触3、4、5、6驱动胶质瘤进展。神经细胞和胶质瘤细胞之间的谷氨酸能AMPA受体依赖突触已在儿童和成人高级别胶质瘤中得到证实,早期研究表明胶质瘤中存在异质性的gaba能反应。然而,除谷氨酸外,神经递质介导的神经元到胶质瘤突触仍未得到充分研究。利用全细胞膜片钳电生理学、体内光遗传学和患者来源的原位异种移植模型,我们鉴定了DMGs中GABAA受体介导的功能性、促肿瘤的GABAA能神经元到胶质瘤突触。由于NKCC1氯离子转运体的功能,gaba能输入对DMG细胞具有去极化作用,从而导致DMG恶性细胞内氯离子浓度升高。由于膜去极化增加胶质瘤的增殖3,6,我们发现gaba能中间神经元的活性促进了DMG在体内的增殖。苯二氮卓类药物劳拉西泮增强gaba介导的信号传导,增加胶质瘤的增殖和生长,缩短DMG患者来源的原位异种移植模型的生存期。相比之下,在半球hgg中只发现最小的去极化gaba能电流,劳拉西泮不影响半球胶质母细胞瘤异种移植物的生长速度。总之,这些发现揭示了gaba能神经元和h3k27m改变的DMG细胞之间促进生长的gaba能突触通信,强调了脑癌神经生理学的肿瘤亚型特异性机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

GABAergic neuron-to-glioma synapses in diffuse midline gliomas
High-grade gliomas (HGGs) are the leading cause of brain cancer-related death. HGGs include clinically, anatomically and molecularly distinct subtypes that stratify into diffuse midline gliomas (DMGs), such as H3K27M-altered diffuse intrinsic pontine glioma, and hemispheric HGGs, such as IDH wild-type glioblastoma. Neuronal activity drives glioma progression through paracrine signalling1,2 and neuron-to-glioma synapses3–6. Glutamatergic AMPA receptor-dependent synapses between neurons and glioma cells have been demonstrated in paediatric3 and adult4 high-grade gliomas, and early work has suggested heterogeneous glioma GABAergic responses7. However, neuron-to-glioma synapses mediated by neurotransmitters other than glutamate remain understudied. Using whole-cell patch-clamp electrophysiology, in vivo optogenetics and patient-derived orthotopic xenograft models, we identified functional, tumour-promoting GABAergic neuron-to-glioma synapses mediated by GABAA receptors in DMGs. GABAergic input has a depolarizing effect on DMG cells due to NKCC1 chloride transporter function and consequently elevated intracellular chloride concentration in DMG malignant cells. As membrane depolarization increases glioma proliferation3,6, we found that the activity of GABAergic interneurons promotes DMG proliferation in vivo. The benzodiazepine lorazepam enhances GABA-mediated signalling, increases glioma proliferation and growth, and shortens survival in DMG patient-derived orthotopic xenograft models. By contrast, only minimal depolarizing GABAergic currents were found in hemispheric HGGs and lorazepam did not influence the growth rate of hemispheric glioblastoma xenografts. Together, these findings uncover growth-promoting GABAergic synaptic communication between GABAergic neurons and H3K27M-altered DMG cells, underscoring a tumour subtype-specific mechanism of brain cancer neurophysiology. Functional, tumour-promoting GABAergic neuron-to-glioma synapses in diffuse midline gliomas are identified.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


