双链断裂时DNA复制局部衰减的机制
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
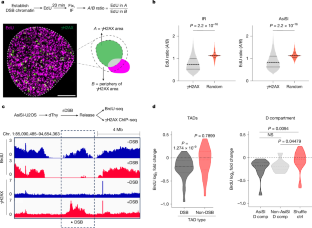
DNA双链断裂(DSBs)破坏了基因组的连续性,导致恶性转化。大量的DNA损伤会引发细胞检查点反应,阻止细胞增殖1,2。然而,高侵袭性的癌细胞能够容忍广泛的DNA损伤,如何对dsb和连续的染色体复制做出反应尚不清楚。本研究表明,dsb诱导了一种局部基因组维持机制,抑制了包含dsb的拓扑相关结构域(TADs)的复制起始,而不影响其他基因组位置的DNA合成。这一过程是由复制和dsb (mrd)的介质促进的。在正常细胞和癌细胞中,mrd包括timless - tipin复合物和WEE1激酶,它们主动地将timless - tipin复合物从dsb附近的复制起点移除,并阻止含有dsb的TADs的DNA合成起始。mrd的失调,或通过溶解TADs破坏三维染色质结构,会导致受损染色质的无意复制和癌细胞中DNA损伤的增加。我们提出,完整的MRD级联先于DSB修复,以防止基因组不稳定,否则当复制被强迫时,或当基因组结构受到挑战时,dsb3,4,5。这些观察结果揭示了DNA复制机制中先前未知的脆弱性,可能被用于治疗靶向癌细胞。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Mechanism for local attenuation of DNA replication at double-strand breaks
DNA double-strand breaks (DSBs) disrupt the continuity of the genome, with consequences for malignant transformation. Massive DNA damage can elicit a cellular checkpoint response that prevents cell proliferation1,2. However, how highly aggressive cancer cells, which can tolerate widespread DNA damage, respond to DSBs alongside continuous chromosome duplication is unknown. Here we show that DSBs induce a local genome maintenance mechanism that inhibits replication initiation in DSB-containing topologically associating domains (TADs) without affecting DNA synthesis at other genomic locations. This process is facilitated by mediators of replication and DSBs (MRDs). In normal and cancer cells, MRDs include the TIMELESS–TIPIN complex and the WEE1 kinase, which actively dislodges the TIMELESS–TIPIN complex from replication origins adjacent to DSBs and prevents initiation of DNA synthesis at DSB-containing TADs. Dysregulation of MRDs, or disruption of 3D chromatin architecture by dissolving TADs, results in inadvertent replication in damaged chromatin and increased DNA damage in cancer cells. We propose that the intact MRD cascade precedes DSB repair to prevent genomic instability, which is otherwise observed when replication is forced, or when genome architecture is challenged, in the presence of DSBs3–5. These observations reveal a previously unknown vulnerability in the DNA replication machinery that may be exploited to therapeutically target cancer cells. DNA double-strand breaks induce local genome maintenance and inhibition of replication initiation at nearby topologically associating domains without affecting global DNA synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


