光化学相互作用:照亮药物传递和诊断的道路
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
Light是一种与尖端纳米技术相结合的非侵入性工具,在基于成像的诊断和癌症和细菌治疗的药物输送方面取得了革命性的进步。本文讨论了这些领域的最新进展,从新兴的成像技术开始。与由可见光激活的传统光传感器不同,替代能源如近红外(NIR)光、x射线和超声波已经被广泛研究,以激活各种光传感器,实现高灵敏度、波长通用性和深度组织成像的空间分辨率。此外,为了解决实时荧光成像中的组织自身荧光等挑战,余辉发光纳米粒子正在被开发,通过整合这些替代能源,在深层组织中进行实时成像和传感,以精确诊断和治疗表面组织以外的癌症。除了深层组织成像,光响应纳米药物通过实现空间和时间控制药物释放,正在彻底改变抗癌和抗菌光疗。这些智能纳米颗粒被设计成针对肿瘤或感染的特定微环境信号,在目标部位释放治疗货物。在抗癌光疗中,这些纳米颗粒通过光异构化、光热和光动力过程促进药物的受控释放。为了延长循环时间和提高靶向性,模拟人体自然抗肿瘤反应的仿生纳米颗粒越来越受到人们的关注。在抗菌光疗中,研究一直集中在光敏剂的化学修饰上,以实现靶向药物递送。最近出现了一种有趣的策略,涉及“前光敏剂”的开发,该策略在光照射下在细菌细胞内被特异性激活,提供了很高的安全性。这些进步利用光化学反应和纳米技术来提高解决关键健康挑战的精确治疗和诊断。本文章由计算机程序翻译,如有差异,请以英文原文为准。

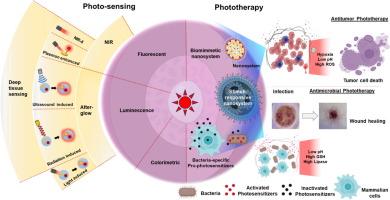
PhotoChem Interplays: Lighting the Way for Drug Delivery and Diagnosis
Light, a non-invasive tool integrated with cutting-edge nanotechnologies, has driven transformative advancements in imaging-based diagnosis and drug delivery for cancer and bacterial treatments. This review discusses recent progress in these areas, beginning with emerging imaging technologies. Unlike traditional photosensors activated by visible light, alternative energy sources such as near-infrared (NIR) light, X-rays, and ultrasound have been extensively investigated to activate various photosensors, achieving high sensitivity, wavelength versatility, and spatial resolution for deep-tissue imaging. Moreover, to address challenges like tissue autofluorescence in real-time fluorescence imaging, afterglow luminescent nanoparticles are being developed by integrating these alternative energy sources for real-time imaging and sensing in deep tissue for precise cancer diagnosis and treatment beyond superficial tissues. In addition to deep tissue imaging, light-responsive nanomedicines are revolutionizing anticancer and antimicrobial phototherapy by enabling spatially and temporally controlled drug release. These smart nanoparticles are engineered to release therapeutic cargo at target sites in response to microenvironmental cues specific to tumors or infections. In anticancer phototherapy, these nanoparticles facilitate controlled drug release via photoisomerization, photothermal, and photodynamic processes. To enhance circulation time and specific targeting, biomimetic nanoparticles, which mimic natural anti-tumor responses by our body, have attracted increasing attention. In antimicrobial phototherapy, research has been focused on the chemical modification of the photosensitizer to enable targeted drug delivery. An intriguing strategy has recently emerged involving the development of “pro-photosensitizers” that are specifically activated within bacterial cells upon light irradiation, offering a high margin of safety. These advancements leverage photochemical reactions and nanotechnology to enhance precision therapy and diagnosis in addressing critical health challenges.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


