基于DNA四面体的FRET纳米传感器用于观察阿尔茨海默病小鼠模型PLD3波动
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
越来越多的证据支持磷脂酶D3 (PLD3)在阿尔茨海默病(AD)发病机制中的重要作用,但PLD3在AD中的实际表达水平和分布仍存在争议。开发特定的纳米探针可能是更好地了解PLD3的一个有前途的策略,但是在这个领域,用于简单、可靠和生物相容性的生物传感器的方法有限。在这项工作中,我们报道了一种利用四面体DNA纳米结构(TDNs)的PLD3诱导荧光共振能量转移(FRET)纳米探针,用于观察AD患者器官和亚细胞水平PLD3的波动。PLD3水解成TDN上的特定核苷酸链将使FRET探针处于关闭状态,从而导致荧光强度的变化。脑切片免疫荧光染色证实了TDN纳米探针可见PLD3的可靠性,并在AD小鼠中观察到PLD3的上调。随后应用纳米探针首次在AD小鼠心脏组织中发现PLD3。在细胞水平上的进一步研究表明,TDN纳米探针与正常神经元中溶酶体的共定位良好,而它们的荧光信号与线粒体的重叠比与AD神经元中溶酶体的重叠更好。我们的发现不仅提供了对PLD3的见解,而且还提供了tdn在多层次AD机制研究中的鼓舞人心的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
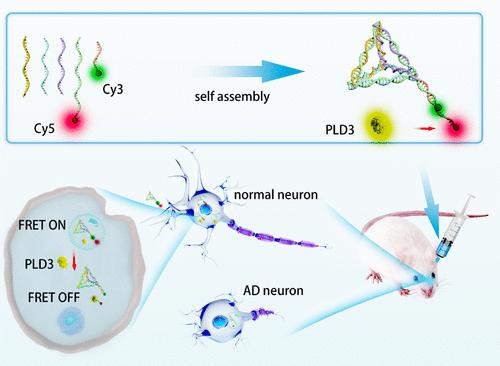
FRET Nanosensor Based on DNA Tetrahedron for Visualizing PLD3 Fluctuation in Mouse Models of Alzheimer’s Disease
Accumulating evidence supports an important role of phospholipase D3 (PLD3) in the pathogenesis of Alzheimer’s disease (AD), while the actual expression level and distribution of PLD3 remains controversial in AD. Developing specific nanoprobes could be a promising strategy to understand PLD3 better, but there are limited approaches available in this field for a simple, reliable, and biocompatible biosensor. In this work, we report a PLD3-induced fluorescence resonance energy transfer (FRET) nanoprobe utilizing tetrahedral DNA nanostructures (TDNs) for visualizing the fluctuation of PLD3 at organ and subcellular levels in AD. Hydrolysis of PLD3 to a specific nucleotide strand on TDN will turn the FRET probe to an OFF state, which results in changes in fluorescent intensity. Immunofluorescent staining of brain sections proved the reliability of TDN nanoprobe to visualize PLD3 and the upregulation of PLD3 was observed in AD mice. Subsequent application of the nanoprobe uncovered PLD3 in the heart tissue of AD mice for the first time. Further investigations on the cellular level revealed a good colocalization of TDN nanoprobes with lysosomes in normal neurons, while their fluorescent signal overlaps better with mitochondria than lysosomes in AD neurons. Our finding provides not only insights into PLD3 but also an inspiring application of TDNs in the mechanism research of AD at multiple levels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


