IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
尺寸可控的超细金属基催化剂的合成对于化学转化技术至关重要。本研究提出了一种空间约束策略,用于合成具有超细 Rh 纳米颗粒(1-3 nm)的 Rh/CeO2-ZrO2 (0.5 wt % Rh) 三向催化剂。该策略利用了 Rh 离子与 CeO2-ZrO2 表面之间强大的静电吸附作用,以及在 Rh 粒子生长过程中由载体介孔提供的空间阻碍所产生的自约束效应。在一氧化碳(CO)、碳氢化合物(HCs)和一氧化氮(NO)混合气体条件下的三元催化反应中,粒径为 2.19 纳米的纳米颗粒催化剂(NPC)表现出很高的催化性能,超过了 Rh 单原子催化剂(SAC)和其他不同 Rh 粒径的 NPC。与 Rh NPC 相比,Rh SAC 在不含 NO 气体分子的反应气氛中显示出更高的 CO 氧化活性和相当的 C3H6 氧化活性。然而,NO 分子的存在阻碍了 CO 和 HC 在 Rh 单原子位点上的吸附和反应。由于 Rh 纳米粒子具有多原子活性中心结构,因此 NO 对 Rh NPC 的影响较弱,从而增强了在三向反应气氛中的低温催化活性。此外,在水热老化条件下,NPC 比 SAC 具有更好的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
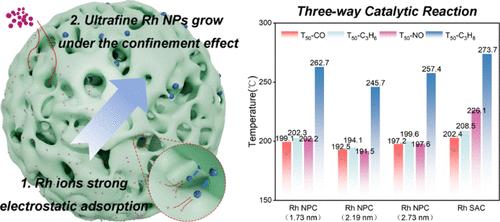
Ultrafine Nanoparticle Rh/CeO2–ZrO2 Catalysts Synthesized via Spatial Confinement: Higher Three-Way Catalytic Activity Compared to Rh Single-Atom Catalyst
The synthesis of size-controlled ultrafine metal-based catalysts is vitally important for chemical conversion technologies. This study presents a spatial confinement strategy for the synthesis of Rh/CeO2–ZrO2 (0.5 wt % Rh) three-way catalysts with ultrafine Rh nanoparticles (1–3 nm). This strategy utilizes the self-confinement effect of Rh ions through the strong electrostatic adsorption between Rh ions and the surface of CeO2–ZrO2, as well as the spatial hindrance provided by the mesopores of the support during Rh particle growth. The nanoparticle catalyst (NPC) with a size of ∼2.19 nm exhibits high catalytic performance, surpassing the Rh single-atom catalyst (SAC) and the other NPCs with different Rh sizes in the three-way catalytic reaction under a gas mixture of carbon monoxide (CO), hydrocarbons (HCs), and nitric oxide (NO). Rh SAC displays higher CO oxidation activity and comparable C3H6 oxidation activity compared with Rh NPC in reaction atmospheres without NO gas molecules. However, the presence of NO molecules hinders the adsorption and reaction of CO and HCs on the Rh single-atom sites. The impact of NO on Rh NPC is weaker due to the multiatomic active center structure of the Rh nanoparticles, resulting in enhanced low-temperature catalytic activity in three-way reaction atmospheres. Additionally, NPC demonstrates better stability than SAC under hydrothermal aging condition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


