双平面类小n杂环碳烯在挑战ru催化立体拥挤烯烃复合反应中的有效配体
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
制备位阻烯烃的烯烃复合方法仍然很少。它们通常基于特殊的钌催化剂,具有立体还原的n -杂环碳(NHC)配体,在催化步骤中能够容纳大的烯烃底物。然而,这些配合物很容易在NHC n -芳基的邻位发生分子内C-H活化,导致其分解。考虑到这个有害的过程需要NHC的一个n -芳基臂的旋转,我们在配体中引入了第二层芳香基团。这种位阻导致了更稳定和高效的催化剂,用于挑战立体拥挤烯烃的复分解反应。通过实验测定所得NHC配体的立体电子性质,并通过密度泛函理论(DFT)计算“翅膀”之间通过空间相互作用的性质以及这些配合物的分解途径,这些上层芳香“翅膀”的有益作用得到了合理化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
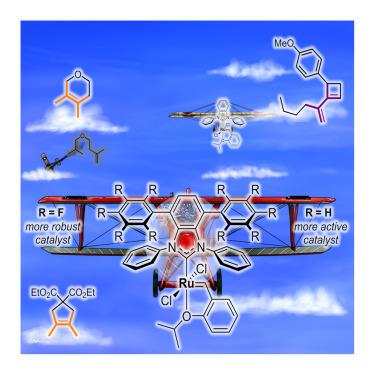
Biplane-like small N-heterocyclic carbenes as effective ligands in challenging Ru-catalyzed metathesis of sterically crowded olefins
Olefin metathesis methods for preparing sterically hindered alkenes remain scarce. They are commonly based on specialized ruthenium catalysts with sterically reduced N-heterocyclic carbene (NHC) ligands, which are able to accommodate large olefinic substrates during the catalytic steps. Yet, these complexes easily undergo intramolecular C–H activation at the ortho position of the N-aryl group of the NHC, which leads to their decomposition. Considering that this deleterious process requires the rotation of one of the NHC’s N-aryl arms, we introduced a second decker of aromatic groups into this ligand. Such steric blockade led to more stable and highly efficient catalysts for challenging metathesis reactions of sterically crowded olefins. The beneficial effect of these upper aromatic “wings” is rationalized through experimental determination of the stereoelectronic properties of the resulting NHC ligands, complemented by density functional theory (DFT) calculations on the nature of the through-space interactions between the “wings” and on the decomposition pathway of these complexes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


