跨位点介导的膜界面螺旋插入的序列依赖尺度
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
Science Advances
Pub Date : 2025-02-19
引用次数: 0
摘要
生物膜是由脂质双分子层组成,上面布满了整体膜蛋白和外周膜蛋白。大多数α-螺旋膜蛋白需要被称为转座子的蛋白质传导插入酶来协助它们的膜插入和折叠。虽然螺旋通过易位或插入膜的序列依赖倾向已被编码为数值疏水性尺度,但相应的分裂到膜界面的倾向仍未揭示。通过在插入宿主蛋白的测试肽序列周围设计诊断糖基化位点,我们设计了一个系统,可以根据其糖基化模式区分序列的水溶性、表面结合和跨膜(TM)状态。使用该系统,我们确定了从易位转移到TM、界面或膜外空间的序列依赖倾向,并将这些倾向与从实验确定的蛋白质的序列和结构确定的相应概率分布进行了比较。本文章由计算机程序翻译,如有差异,请以英文原文为准。
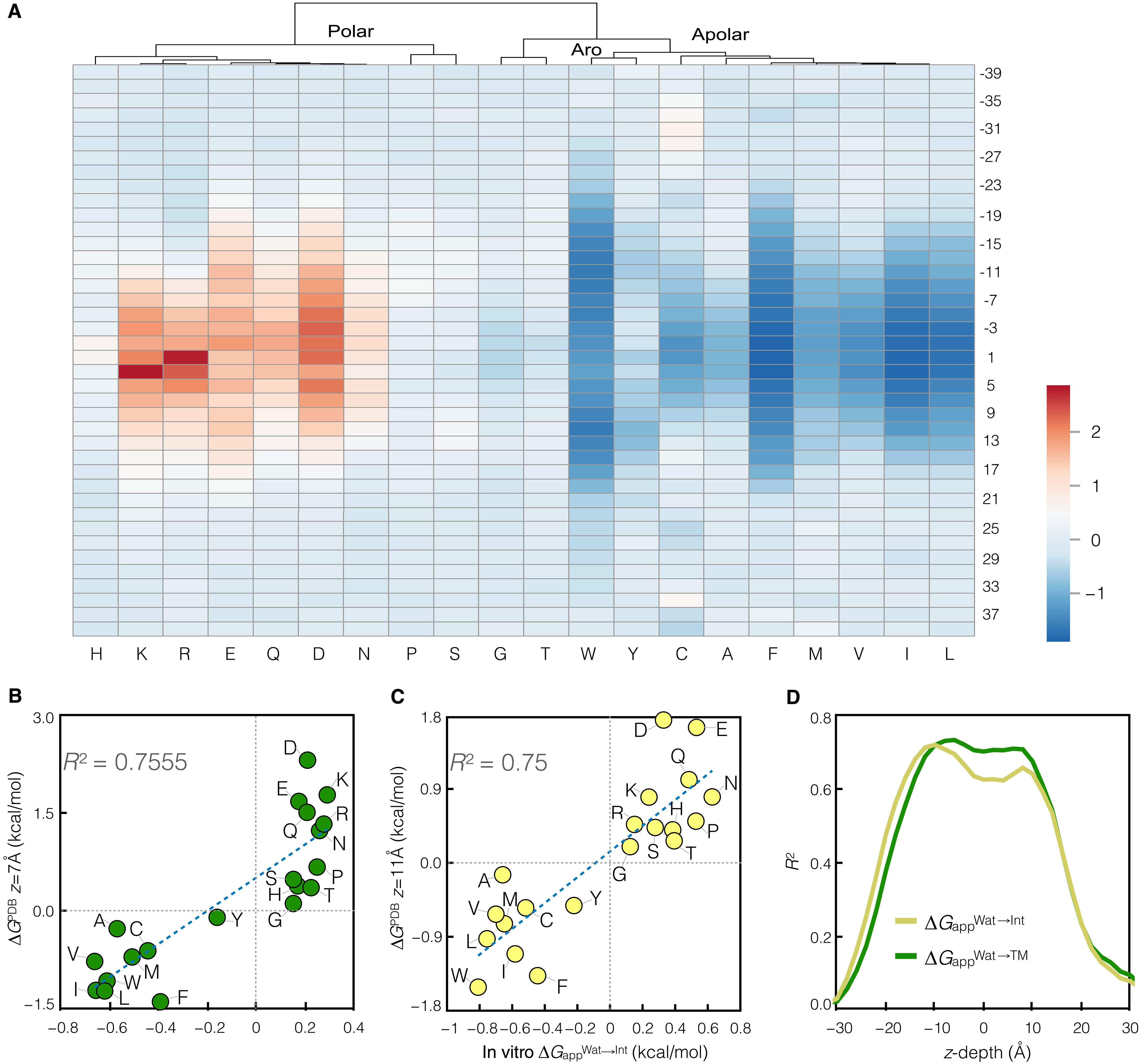
Sequence-dependent scale for translocon-mediated insertion of interfacial helices in membranes
Biological membranes consist of a lipid bilayer studded with integral and peripheral membrane proteins. Most α-helical membrane proteins require protein-conducting insertases known as translocons to assist in their membrane insertion and folding. While the sequence-dependent propensities for a helix to either translocate through the translocon or insert into the membrane have been codified into numerical hydrophobicity scales, the corresponding propensity to partition into the membrane interface remains unrevealed. By engineering diagnostic glycosylation sites around test peptide sequences inserted into a host protein, we devised a system that can differentiate between water-soluble, surface-bound, and transmembrane (TM) states of the sequence based on its glycosylation pattern. Using this system, we determined the sequence-dependent propensities for transfer from the translocon to a TM, interfacial, or extramembrane space and compared these propensities with the corresponding probability distributions determined from the sequences and structures of experimentally determined proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


