外电作用下Ni4/ZrO2(101)催化剂上甲烷干重整机理的综合理论研究:界面和氧空位的作用
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
尽管甲烷干重整反应在环境和经济方面具有优势,但它仍然面临着碳沉积引起的催化剂失活的挑战。金属负载型催化剂在DRM反应中表现出较高的活性和稳定性,这是由于金属负载型催化剂对Ni纳米颗粒的强相互作用(SMSI)和独特的界面活性位点的能力。此外,外加电场对DRM有显著的影响。本文采用密度泛函理论(DFT)和微动力学模型对不同电场条件下Ni4/ZrO2(101)催化剂上DRM反应的机理进行了全面研究,以更好地理解金属支撑界面和氧空位在催化中的重要性。结果表明,电场削弱了Ni4簇与ZrO2(101)之间的相互作用。物质在界面上比只在Ni位点上更容易被吸附和活化。在界面和Ni位点,负电场增强CO2活化,而正电场促进甲烷活化和CHx氧化。负电场下Ni4/ZrO2催化剂在H溢出形成水的过程中产生氧空位。界面和氧空位显著提高了DRM反应活性,减少了碳沉积。表面吸附偶极矩反映了吸附强度和反应焓随电场变化的趋势。微动力学结果表明,Ni4簇上容易形成积碳,以CH-CH为主要积碳类型。幸运的是,在Ni簇和Zr界面的协同作用下,DRM反应表现出较高的反应物转化率。此外,氧空位促进CO2活化,从而减少碳沉积,提高DRM反应活性,特别是在负电场的作用下。正电场对Ni4/ZrO2的DRM反应活性最高,且碳沉积较多。重要的是,负电场结合了高活性和抗碳沉积特性。我们的结果与实验高度一致。这项工作强调了界面和氧空位的重要性,为深入理解DRM反应机理和电场催化领域的研究提供了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
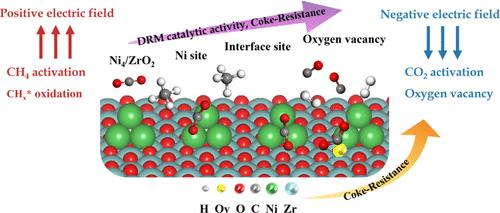
A Comprehensive Theoretical Study of the Mechanism for Dry Reforming of Methane on a Ni4/ZrO2(101) Catalyst Under External Electric Fields: The Role of Interface and Oxygen Vacancy
Although dry reforming of methane (DRM) reactions is advantageous in environmental and economic aspects, they still face the challenge of catalyst deactivation caused by carbon deposition. Metal-supported catalysts exhibit high activity and stability in DRM reactions due to the ability of strong metal–support interaction (SMSI) to highly disperse Ni nanoparticles and unique interface active sites. Moreover, the external electric field has a significant effect on DRM. Herein, the mechanisms of the DRM reaction on the Ni4/ZrO2(101) catalyst under different electric fields were comprehensively investigated using density functional theory (DFT) and microkinetic modeling to better understand the importance of metal-support interfaces and oxygen vacancies in catalysis. The results indicated that the electric fields weaken the interaction between Ni4 clusters and ZrO2(101). Species are more easily adsorbed and activated at the interface than at only Ni sites. The negative electric fields enhanced CO2 activation, while the positive electric fields promoted methane activation and CHx oxidation, at either the interface or Ni sites. Oxygen vacancies were generated on the Ni4/ZrO2 catalysts by H spillover to form water and were facilitated by negative electric fields. The interface and oxygen vacancies significantly enhance DRM reaction activity and reduce carbon deposition. The surface adsorption dipole moment reflects the tendency of the adsorption strength and reaction enthalpy to change with the electric fields. The microkinetic results showed that carbon deposition is easily formed on the Ni4 cluster, with CH–CH being the predominant type of carbon deposition. Fortunately, the DRM reaction exhibits high reactant conversion under the synergistic effect of Ni clusters and the Zr interface. In addition, oxygen vacancies promote CO2 activation, thereby reducing carbon deposition and enhancing DRM reaction activity, especially with the application of a negative electric field. The positive electric field has the highest DRM reaction activity on Ni4/ZrO2, accompanied by more carbon deposition. Importantly, the negative electric field combines high activity and anticarbon deposition properties. Our results are highly consistent with the experiment. This work emphasizes the importance of interfaces and oxygen vacancies, providing theoretical foundations for a deeper understanding of DRM reaction mechanisms and research in the area of electric- field catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


