促进肺恢复:采用先进的吸入塔技术,吸入包封FTY720和诺比列素的聚乳酸-共乙醇酸治疗脂多糖性肺损伤
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
急性肺损伤(ALI)/急性呼吸窘迫综合征(ARDS)是一种进展迅速的呼吸衰竭,死亡率高,尤其是重症。许多试验研究了各种药物治疗方法,但其有效性仍不确定。本研究中,我们提出了一种吸入型芬廖莫德(FTY720)-诺比列素(NOB)-聚乳酸-羟基乙酸(PLGA)纳米颗粒(NPs)的纳米制剂,具有良好的生物相容性和缓释药理作用。该配方降低了FTY720的毒性,并提高了NOB的生物利用度,因为我们使用了具有高生物相容性的PLGA同时包裹FTY720和NOB。在体外,与纯药物治疗相比,我们证明FTY720-NOB-PLGA NPs可以更有效地减少脂多糖(LPS)刺激后巨噬细胞释放的白细胞介素-6 (IL-6)和活性氧(ROS)。在体内,我们使用吸入塔系统,使未麻醉的小鼠在受控条件下暴露于雾化的FTY720-NOB-PLGA NPs。我们证明吸入FTY720-NOB-PLGA NPs可以通过抑制细胞因子的释放,如IL-6和肿瘤坏死因子-α (TNF-α),减轻LPS暴露后的肺损伤。ALI的触发通路,包括活化B细胞核因子κ-轻链增强子(NF-κB)和p38丝裂原活化蛋白激酶也被有效抑制。此外,吸入治疗提供了良好的安全性,对生化指标和肺功能没有有害影响。我们提供了无创吸入NPs并持续监测肺功能的可行性。我们开发的雾化FTY720-NOB-PLGA NPs在未来的急性肺损伤治疗中显示出良好的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
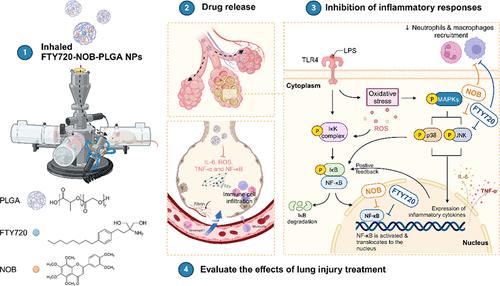
Enhancing Lung Recovery: Inhaled Poly(lactic-co-glycolic) Acid Encapsulating FTY720 and Nobiletin for Lipopolysaccharide-Induced Lung Injury, with Advanced Inhalation Tower Technology
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), a rapidly progressing respiratory failure condition, results in a high mortality rate, especially in severe cases. Numerous trials have investigated various pharmacotherapy approaches, but their effectiveness remains uncertain. Here, we present an inhaled nanoformulation of fingolimod (FTY720)-nobiletin (NOB)- poly(lactic-co-glycolic) acid (PLGA) nanoparticles (NPs) with good biocompatibility and a sustained-release pharmacological effect. The formulation decreases the toxicity of FTY720 and increases the bioavailability of NOB since we use PLGA with a high biocompatibility to encapsulate FTY720 and NOB at the same time. In vitro, in comparison to treatment with the pure drug, we demonstrated that FTY720-NOB-PLGA NPs can reduce interleukin-6 (IL-6) and reactive oxygen species (ROS) release by macrophages after lipopolysaccharide (LPS) stimulation more efficiently. In vivo, we used an inhalation tower system that allowed the exposure of unanesthetized mice to aerosolized FTY720-NOB-PLGA NPs under controlled conditions. We demonstrated that inhaled FTY720-NOB-PLGA NPs can attenuate lung injury after LPS exposure by suppressing cytokine release, such as IL-6 and tumor necrosis factor-α (TNF-α). The trigger pathway of ALI, including nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and p38 mitogen-activated protein kinase, was also efficiently inhibited. Furthermore, the inhalation treatment provided a good safety profile, without detrimental effects on biochemical markers and lung function. We provided the feasibility of administering inhalation of NPs noninvasively with continuous monitoring of lung function. The aerosolized FTY720-NOB-PLGA NPs we developed show excellent promise for acute lung injury therapy in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


