拟南芥根系微生物群中黄菌共生菌对宿主关联的保守免疫调节和变异
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
慢性拟南芥免疫反应的抑制是植物微生物群主要细菌谱系中普遍存在的,但通常是菌株特异性的特征。我们通过系统发育分析和代表性菌株的植物关联表明,免疫调节在黄单胞菌属中是一个高度保守的祖先特征,并且在这些细菌作为宿主适应病原体特化之前。罗丹诺杆菌R179激活免疫应答,但根转录组学表明,这种共生体在长期关联时逃避宿主免疫感知。R179的伪装可能是由于两种转运体复合物(dssAB)的联合活性和来自所有伙伴的免疫原性肽的选择性消除。R179在植物免疫系统中掩盖自身和其他共生体的能力,与共接种R179时由免疫抑制性或非抑制性合成微生物群引发的不同根转录组的趋同一致。通过dssAB的免疫调节使R179在根室的合成群落中具有竞争优势。我们认为,黄单胞菌的广泛免疫调节与它们对陆地栖息地的适应有关,并可能导致菌株特异性根关联的变化,这些变化共同说明了它们在植物微生物群建立中的重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

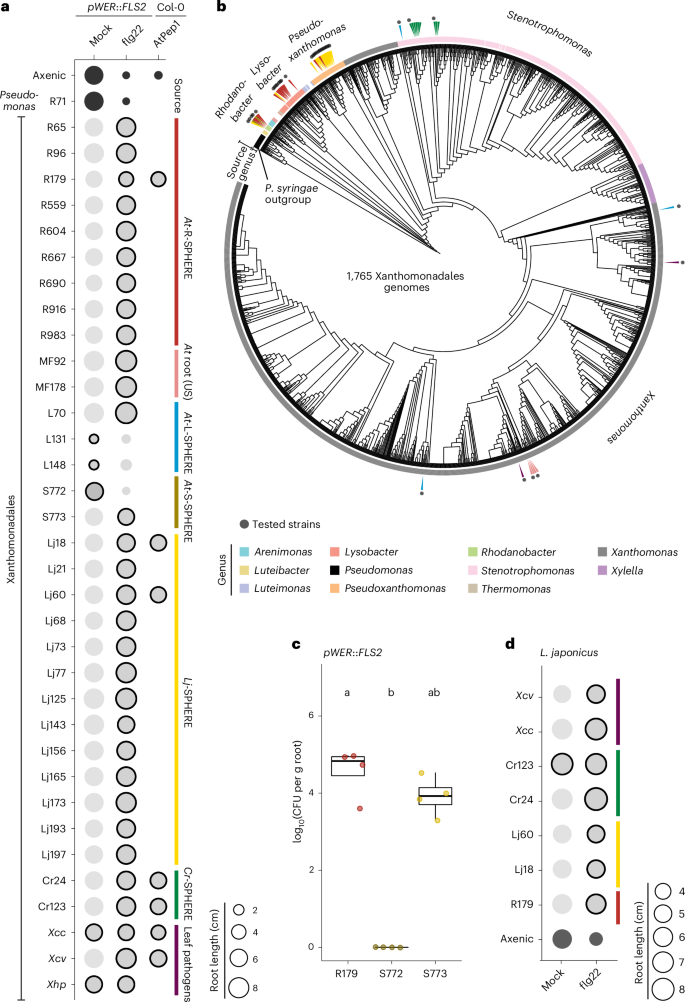
Conserved immunomodulation and variation in host association by Xanthomonadales commensals in Arabidopsis root microbiota
Suppression of chronic Arabidopsis immune responses is a widespread but typically strain-specific trait across the major bacterial lineages of the plant microbiota. We show by phylogenetic analysis and in planta associations with representative strains that immunomodulation is a highly conserved, ancestral trait across Xanthomonadales, and preceded specialization of some of these bacteria as host-adapted pathogens. Rhodanobacter R179 activates immune responses, yet root transcriptomics suggest this commensal evades host immune perception upon prolonged association. R179 camouflage likely results from combined activities of two transporter complexes (dssAB) and the selective elimination of immunogenic peptides derived from all partners. The ability of R179 to mask itself and other commensals from the plant immune system is consistent with a convergence of distinct root transcriptomes triggered by immunosuppressive or non-suppressive synthetic microbiota upon R179 co-inoculation. Immunomodulation through dssAB provided R179 with a competitive advantage in synthetic communities in the root compartment. We propose that extensive immunomodulation by Xanthomonadales is related to their adaptation to terrestrial habitats and might have contributed to variation in strain-specific root association, which together accounts for their prominent role in plant microbiota establishment. The authors show that immunosuppression is highly conserved in the bacterial order Xanthomonadales. This feature, which preceded their specialization as host-adapted pathogens, probably contributes to their prominence as core members of the plant microbiota.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


