肠隐窝微生物群通过吲哚乙酸调节肠干细胞的更新和肿瘤发生
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
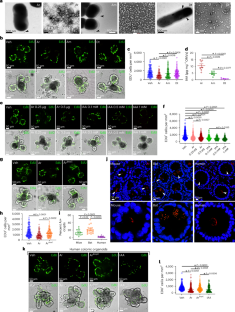
肠隐窝窝藏着特定的微生物群,但这些细菌是否以及如何调节肠干细胞或影响结直肠癌(CRC)的发展尚不清楚。在这里,我们筛选了类器官中的隐窝细菌,发现由耐辐射不动杆菌分泌的吲哚乙酸(IAA)抑制ISC的转换。A.放射耐药抑制结直肠癌患者肿瘤切片中的细胞增殖,抑制APCMin/+小鼠肠道肿瘤发生和球形起始。在结直肠癌小鼠模型中,利用噬菌体靶向清除结肠隐窝中的放射耐药芽孢杆菌可增加EphB2的表达,从而促进细胞增殖、ISC转换和肿瘤发生。通过删除trpC以阻止IAA的产生,或敲除肠道特异性芳烃受体(AhR),确定了促进ISC稳态的IAA-AhR- wnt -β-catenin信号轴,从而消除了辐射耐药芽胞杆菌的保护作用。我们的研究结果揭示了肠道隐窝微生物群成员在肿瘤发生中的保护作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Intestinal crypt microbiota modulates intestinal stem cell turnover and tumorigenesis via indole acetic acid
Intestinal crypts harbour a specific microbiota but whether and how these bacteria regulate intestinal stem cells (ISCs) or influence colorectal cancer (CRC) development is unclear. Here we screened crypt-resident bacteria in organoids and found that indole acetic acid (IAA) secreted by Acinetobacter radioresistens inhibits ISC turnover. A. radioresistens inhibited cellular proliferation in tumour slices from CRC patients and inhibited intestinal tumorigenesis and spheroid initiation in APCMin/+ mice. Targeted clearance of A. radioresistens from colonic crypts using bacteriophage increased EphB2 expression and consequently promoted cellular proliferation, ISC turnover and tumorigenesis in mouse models of CRC. The protective effects of A. radioresistens were abrogated upon deletion of trpC to prevent IAA production, or upon intestine-specific aryl hydrocarbon receptor (AhR) knockout, identifying an IAA-AhR-Wnt-β-catenin signalling axis that promotes ISC homeostasis. Our findings reveal a protective role for an intestinal crypt-resident microbiota member in tumorigenesis. Intestinal crypt-resident Acinetobacter radioresistens secretes indole acetic acid which activates an Ahr-Wnt-β-catenin-EphB2 signalling axis to promote intestinal stem cell homeostasis and prevent tumour initiation and colorectal cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


