氧还原允许从水纳米液滴电沉积形貌可调的铜纳米颗粒
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
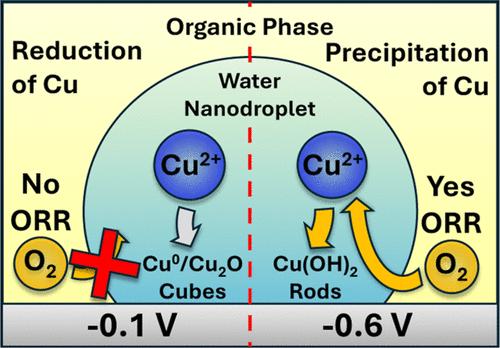
以简单和经济的方式扩大金属纳米颗粒的可调性对于开发未来能量转换系统所需的多相催化剂至关重要。目前许多在不同纳米颗粒形态和组成之间切换的方法包括使用表面活性剂、pH调节或其他共反应物。一种相对未开发的调整纳米颗粒特性的新途径是利用纳米液滴介导电沉积技术中水滴周围的有机相。这些含水纳米液滴含有金属前驱体盐,当它们与充分偏压的电极碰撞时,电沉积纳米颗粒。有机溶剂,如1,2-二氯乙烷,已知与水相比具有相对较高的双氧溶解度,可能在液滴界面提供富氧环境,促进非均相氧还原。在这项工作中,氧还原反应被用于铜的电沉积,以调整所得纳米颗粒的形态和成分。并将这些效应与体水电沉积中的效应进行了比较。通过电化学技术、电子显微镜、能量色散x射线能谱和x射线光电子能谱研究了纳米颗粒的性质和氧还原在其合成中的作用。当只在电极处还原铜时,得到的纳米颗粒具有一系列立方和球形形貌,并具有多种铜氧化态,表明零价铜和氧化铜纳米颗粒。当还原铜和氧时,电沉积的纳米颗粒具有独特的棒状形态,其氧化态和原子比表明氢氧化铜。当铜在相同的还原电位下从大块水溶液中电沉积时,后者的纳米颗粒形态和组成是无法实现的。我们的研究结果表明,人们可以利用发生在水|有机|电极界面上的基本电化学来调整铜纳米粒子的关键性质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Oxygen Reduction Allows Morphology-Tunable Copper Nanoparticle Electrodeposition from Aqueous Nanodroplets
Expanding the tunability of metallic nanoparticles in simple and cost-effective manners is essential for developing heterogeneous catalysts needed for the energy conversion systems of the future. Many current methods of switching between different nanoparticle morphologies and compositions include the use of surfactants, pH adjustments or other coreactants. One relatively unexplored and new route to tuning these nanoparticle properties involves taking advantage of the organic phase surrounding the aqueous droplets used in nanodroplet mediated electrodeposition techniques. These aqueous nanodroplets contain metal precursor salts that electrodeposit nanoparticles when they collide with a sufficiently biased electrode. Organic solvents such as 1,2-dichloroethane, known to have relatively high dioxygen solubilities compared to water, may provide an oxygen rich environment at the droplet interface, promoting heterogeneous oxygen reduction. In this work, the oxygen reduction reaction is used in the electrodeposition of copper to tune the resulting nanoparticle morphologies and compositions. These effects are also compared to those in bulk aqueous electrodeposition. The properties of the nanoparticles and the role of oxygen reduction in their synthesis are probed through electrochemical techniques, electron microscopy, energy dispersive X-ray spectroscopy, and X-ray photoelectron spectroscopy. When only reducing copper at the electrode, the resulting nanoparticles possess a range of cubic and spherical morphologies and multiple copper oxidation states indicative of zerovalent copper and copper oxide nanoparticles. When reducing both copper and oxygen, the electrodeposited nanoparticles possess a distinctive rod-like morphology with oxidation states and atomic ratios indicative of copper hydroxide. The latter nanoparticle morphology and composition was not attainable when copper was electrodeposited from a bulk aqueous solution at the same applied reducing potential. Our results show that one can take advantage of the fundamental electrochemistry taking place at the aqueous|organic|electrode interface to tune key properties of copper nanoparticles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


