尼龙-66解聚对循环经济的影响:动力学建模、净化和可持续设计
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
尼龙-66是一种重要的热塑性塑料,广泛应用于汽车零部件、电子产品和纺织品。化学解聚生成尼龙-66单体,即己二酸(AA)和己二胺(HMDA),为低纯度和降解的尼龙-66废物提供了一种有效的回收价值的方法。虽然阶梯生长聚合物(如PET和尼龙-6)的化学回收途径已被广泛研究,但尼龙-66的化学回收尚不清楚。这项工作提出了一个全面的评估学术文献和工业专利的三种主要类型的尼龙-66解聚过程:酸水解,碱性水解和氨解。我们利用现有数据建立了一个动力学模型,该模型包含了尼龙-66中性水解的活度系数,以反映具有高浓度水的非理想液相,并且我们将降解反应纳入模型副产物。我们确定了AA、HDMA、氨解产物和尼龙66盐的净化方法,并描述了它们如何应用于化学回收途径。我们提出了尼龙-66碱性水解的第一个过程模型,利用PET碱性水解的创新,展示了热集成和过程强化,如机械蒸汽再压缩。我们证明了尼龙-66的碱性水解工艺比PET碱性水解工艺消耗更少的能量,同时生产出更高的产品价值。我们利用这一集体评价为今后进一步推进尼龙66化学回收的研究提供指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
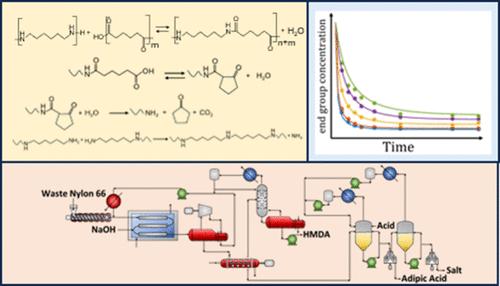
Assessment of Nylon-66 Depolymerization for Circular Economy: Kinetic Modeling, Purification, and Sustainable Design
Nylon-66 is an important thermoplastic that finds widespread applications in automotive parts, electronics, and textiles. Chemical depolymerization to form nylon-66 monomers, namely, adipic acid (AA) and hexamethylene diamine (HMDA), offers an efficient method to recover value from low-purity and degraded nylon-66 waste. While chemical recycling pathways for step-growth polymers, such as PET and nylon-6, have been extensively investigated, the chemical recycling of nylon-66 is not well understood. This work presents a comprehensive assessment of the academic literature and industrial patents of the three primary types of nylon-66 depolymerization processes: acid hydrolysis, alkaline hydrolysis, and ammonolysis. We use existing data to develop a kinetic model incorporating the activity coefficient for neutral hydrolysis of nylon-66 necessary to reflect the nonideal liquid phase with high water concentration, and we include degradation reactions to model byproducts. We identify purification methods for AA, HDMA, ammonolysis products, and nylon-66 salts and describe how they can be applied to chemical recycling pathways. We present the first process model for the alkaline hydrolysis of nylon-66 leveraging innovations from PET alkaline hydrolysis and demonstrating heat integration and process intensifications, such as mechanical vapor recompression. We demonstrate that the alkaline hydrolysis process for nylon-66 consumes less energy than the comparative PET alkaline hydrolysis process, while producing higher-value products. We use this collective evaluation to provide guidance for future research to further advance nylon-66 chemical recycling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


