通过I型蛋白精氨酸甲基转移酶抑制组蛋白精氨酸不对称二甲基化的光表观遗传学调控
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
组蛋白精氨酸不对称二甲基化主要由I型蛋白精氨酸甲基转移酶(PRMTs)催化,涉及广泛的生物学和病理过程。最近,一些I型PRMT抑制剂,如MS023,已被开发用于逆转肿瘤细胞中组蛋白精氨酸二甲基化状态,但I型PRMT的广泛抑制可能会在正常组织中引起副作用。在此,我们设计了一种可光激活的MS023前药(C-MS023)来实现对组蛋白精氨酸不对称二甲基化的时空抑制。体外研究表明,C-MS023抑制I型PRMTs的效力降低。重要的是,420 nm的可见光照射可以触发前药光解,从而释放MS023,有效下调组蛋白精氨酸不对称二甲基化和DNA复制相关的转录组活性。这种光表观遗传小分子前药可能有助于进一步研究I型PRMTs的病理生理功能和靶向表观遗传治疗的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
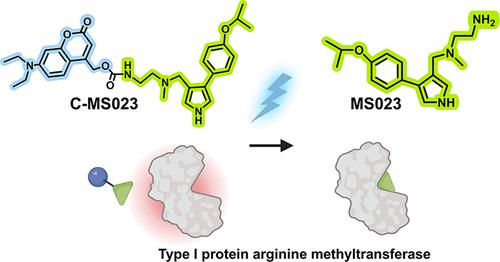
Opto-Epigenetic Regulation of Histone Arginine Asymmetric Dimethylation via Type I Protein Arginine Methyltransferase Inhibition
Histone arginine asymmetric dimethylation, which is mainly catalyzed by type I protein arginine methyltransferases (PRMTs), is involved in broad biological and pathological processes. Recently, several type I PRMT inhibitors, such as MS023, have been developed to reverse the histone arginine dimethylation status in tumor cells, but extensive inhibition of type I PRMTs may cause side effects in normal tissues. Herein, we designed a photoactivatable MS023 prodrug (C-MS023) to achieve spatiotemporal inhibition of histone arginine asymmetric dimethylation. In vitro studies showed that C-MS023 exhibited reduced potency in inhibiting type I PRMTs. Importantly, visible light irradiation at 420 nm could trigger the photolysis of the prodrug, thereby liberating MS023 for effective downregulation of histone arginine asymmetric dimethylation and DNA replication-related transcriptomic activities. This opto-epigenetic small-molecule prodrug potentially aids in further research into the pathophysiological functions of type I PRMTs and the development of targeted epigenetic therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


