由α-甲基磷酸丝氨酸衍生的新型四取代α-氨基膦酸盐的一锅合成及其抗炎作用的体内评价
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
描述了一系列新的带有甲基磷酸丝氨酸片段的四取代α-氨基膦酸衍生物。这些化合物是通过三组分(3-CR)“卡巴尼克场反应”合成的。通过外用和口服给药途径筛选新型α-氨基膦酸盐的体内抗炎活性。所有化合物均以剂量依赖的方式降低tpa诱导的耳部水肿。在本实验中,化合物2、5和7与吲哚美辛具有相同的功效(≈90%)和更高的效力,并能降低炎症标志物中性粒细胞与淋巴细胞的比率(NLR)。此外,与吲哚美辛或(S)-萘普生一样,口服化合物2-7预处理和后处理均可减少cfa诱导的足跖水肿。基于它们在体内具有良好的抗炎效果,我们在计算机上研究了它们的物理化学和药代动力学特征。分析还表明,新型四取代α-氨基膦酸盐不打破Lipinski五法则,具有药物相似性和良好的口服和透皮给药ADME性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
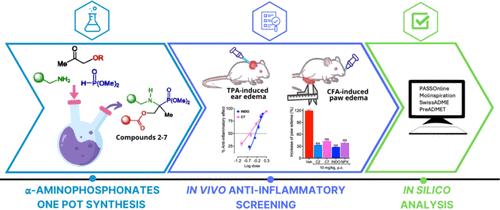
One-Pot Synthesis of Novel Tetrasubstituted α-Aminophosphonates Derived from α-Methylphosphoserine and In Vivo Evaluation as Anti-Inflammatory Agents
A series of new tetrasubstituted α-aminophosphonate derivatives with a methylphosphoserine fragment were described. These compounds were synthesized by a three-component (3-CR) “Kabachnik-Fields reaction.” The novel α-aminophosphonates were screened for in vivo anti-inflammatory activity through topical and oral administration routes. All compounds decreased TPA-induced ear edema in a dose-dependent fashion. In this test, compounds 2, 5, and 7 showed the same efficacy (≈ 90%) and higher potency than indomethacin and decreased the inflammatory marker neutrophil-to-lymphocyte ratio (NLR). Moreover, oral pretreatment and post-treatment with compounds 2–7 reduced CFA-induced paw edema, as did indomethacin or (S)-naproxen. Based on the promising in vivo anti-inflammatory results, we investigated their physicochemical and pharmacokinetics profiles in silico. The analysis also revealed that the novel tetrasubstituted α-aminophosphonates did not break Lipinski’s rule of five and had drug-likeness and favorable ADME properties for oral and transdermal administration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


