声学驱动的混合纳米晶体用于体内胰腺癌治疗
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
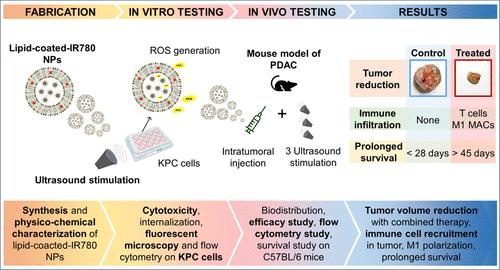
胰腺导管腺癌(pancreatic ductal adencarcinoma, PDAC)是目前最致命的肿瘤之一,迫切需要新的治疗策略。PDAC的特点是缺氧、内在耐药、“冷”的肿瘤微环境和致密的结缔组织增生基质,这阻碍了药物的渗透。本研究探讨了铁掺杂脂质氧化锌纳米颗粒与荧光声敏剂增强和局部超声刺激对PDAC的联合作用。合成纳米颗粒并包被脂质,并通过评估再现性、稳定性和高效包被来表征其物理化学性质。体外,在KPC小鼠PDAC细胞系上联合超声对声敏剂增强纳米结构进行了细胞毒性和疗效评价。在体内,NPs进一步与AlexaFluor 700偶联以允许其随着时间的推移定位,并且纳米构建物被瘤内给药到皮下小鼠PDAC模型中,以提高局部生物利用度和肿瘤可视化,并最大限度地减少全身递送的脱靶效应。在不同的小鼠队列中进行了生物分布、疗效、流式细胞术和生存研究。超声敏化增强的纳米结构,结合超声,引发了大量活性氧(ROS)的产生,降低了KPC细胞的活力。在体内,超声刺激的抗肿瘤效果特别明显,表明纳米颗粒和超声之间存在协同作用。此外,免疫细胞浸润增加,癌细胞凋亡增强,治疗动物存活时间延长。这些发现强调了一种协同治疗方法的潜力,该方法将脂质包被的载声敏剂纳米颗粒和超声刺激结合起来,作为PDAC和原位监测的有效治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acoustically Driven Hybrid Nanocrystals for In Vivo Pancreatic Cancer Treatment
New treatment strategies are urgently needed for pancreatic ductal adenocarcinoma (PDAC), which is one of the deadliest tumors nowadays. PDAC is marked by hypoxia, intrinsic chemoresistance, a “cold” tumor microenvironment, and dense desmoplastic stroma, which hinders drug penetration. This study investigates the combined effect of iron-doped, lipid-coated zinc oxide nanoparticles enhanced with a fluorescent sonosensitizer and local ultrasound stimulation in treating PDAC. Nanoparticles were synthesized and coated by lipids, and their physiochemical properties were characterized by assessing reproducibility, stability, and efficient inclusion of the sonosensitizer. In vitro, sonosensitizer-enhanced nanoconstructs were tested on a KPC murine PDAC cell line in combination with ultrasound to evaluate their cytotoxicity and assess their efficacy. In vivo, NPs were further coupled with AlexaFluor 700 to allow their localization over time, and the nanoconstructs were intratumorally administered to a subcutaneous murine PDAC model to enhance local bioavailability and tumor visualization and minimize off-target effects of systemic delivery. Biodistribution, efficacy, flow cytometry, and survival studies were carried out on different cohorts of mice. The sonosensitizer-enhanced nanoconstructs, combined with ultrasound, triggered significant reactive oxygen species (ROS) production, reducing the KPC cell viability. In vivo, the antitumor efficacy was particularly pronounced with ultrasound stimulation, demonstrating a synergistic interaction between the nanoparticles and ultrasound. Moreover, increased immune cell infiltration, enhanced cancer cell apoptosis, and prolonged survival of the treated animals were achieved. These findings highlight the potential of a synergistic therapeutic approach combining lipid-coated sonosensitizer-loaded nanoparticles and ultrasound stimulation as an effective therapy for PDAC and in situ monitoring.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


