铁酸锰纳米杂交体通过协同激活树突状细胞和巨噬细胞中的cGAS-STING和NF-κB串扰增强抗肿瘤免疫效应
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
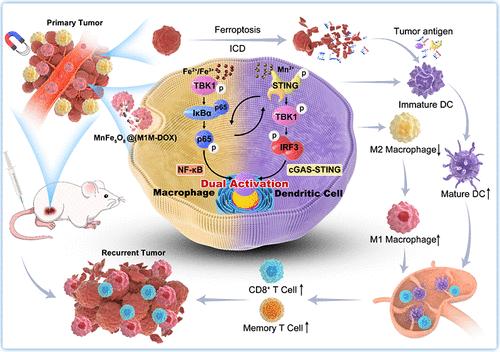
树突状细胞(dc)和肿瘤相关巨噬细胞(tam)的特异性激活可以激活先天和适应性免疫反应,从而逆转肿瘤免疫抑制微环境。在本研究中,合成了锰铁氧体纳米杂化物MnFe5O8@(M1M-DOX)来激活dc和tam中的cGAS-STING和NF-κB串扰。MnFe5O8作为Fe2+/Fe3+和Mn2+的来源,通过M1巨噬细胞细胞膜包裹微剂量的阿霉素(DOX)。Fe2+/Fe3+和DOX可协同诱导肿瘤铁下垂,触发暴露肿瘤抗原的免疫原性细胞死亡(ICD)。Fe2+/Fe3+和Mn2+的释放对dc的激活和tam从M2表型向M1表型的重编程具有内在的双重免疫调节作用。简而言之,Fe2+/Fe3+激活NF-κB信号通路,触发STING信号通路的激活。同时,Mn2+进一步增强STING的激活,并以级联激活的方式刺激NF-κB。因此,cGAS-STING和NF-κB串扰相互增强的双重激活,促使dc和tam强烈成熟,协同促进T细胞浸润,抑制原发肿瘤生长和局部复发。本研究提出了在纳米合金中递送免疫调节金属离子和利用抗原呈递细胞(APCs)中多信号通路的激活的策略,为肿瘤免疫治疗提供了前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Augmenting Antitumor Immune Effects through the Coactivation of cGAS-STING and NF-κB Crosstalk in Dendritic Cells and Macrophages by Engineered Manganese Ferrite Nanohybrids
The specific activation of dendritic cells (DCs) and tumor-associated macrophages (TAMs) can activate innate and adaptive immune responses to reverse the tumor immunosuppressive microenvironment. In this study, manganese ferrite nanohybrid MnFe5O8@(M1M-DOX) is synthesized to activate cGAS-STING and NF-κB crosstalk in DCs and TAMs. MnFe5O8, as the source of Fe2+/Fe3+ and Mn2+, is encapsulated with a microdose of doxorubicin (DOX) using an M1 macrophage cytomembrane. Fe2+/Fe3+ and DOX can cooperatively induce tumorous ferroptosis, triggering immunogenic cell death (ICD) that exposes tumor antigens. The release of Fe2+/Fe3+ and Mn2+ has intrinsic dual-immunomodulatory effects on the activation of DCs and the reprogramming of TAMs from the M2 to M1 phenotype. Briefly, Fe2+/Fe3+ activates the NF-κB signaling pathway to trigger the activation of STING signaling. Meanwhile, Mn2+ further enhances the activation of STING and stimulates NF-κB in a cascade-activating manner. Thus, the mutually reinforcing dual activation of cGAS-STING and NF-κB crosstalk prompts the strong maturation of DCs and TAMs, synergistically promoting the infiltration of T cells to inhibit primary tumor growth and localized recurrence. This work proposes a strategy for delivering immunomodulatory metal ions in nanoalloy and harnessing the activation of multisignaling pathways in antigen-presenting cells (APCs) to provide perspectives for tumor immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


