经批准的cd19靶向CAR - T细胞治疗大B细胞淋巴瘤的疗效相关因素
IF 82.2
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
cd19靶向嵌合抗原受体(CAR) T细胞在治疗复发和/或难治性大B细胞淋巴瘤(LBCL)患者方面取得了突破性进展。目前,三种cd19靶向CAR - T细胞产品已被FDA和其他监管机构批准用于治疗LBCL患者:axicabtagene ciloleucel, tisagenlecleucel和lisocabtagene maraleucel。输注这些cd19靶向CAR - T细胞后的反应率很有希望;然而,大约一半接受治疗的患者在2年内复发。此外,接受这些药物可能与严重的毒性有关,包括细胞因子释放综合征和免疫效应细胞相关的神经毒性综合征。在这篇综述中,我们总结了与疗效相关的因素,包括关键临床试验和描述接受这些产品治疗的LBCL患者结果的大型真实数据集中的cd19靶向CAR - T细胞的反应和生存结果以及毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

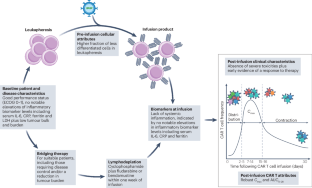
Outcome correlates of approved CD19-targeted CAR T cells for large B cell lymphoma
CD19-targeted chimeric antigen receptor (CAR) T cells have provided a breakthrough in the treatment of patients with relapsed and/or refractory large B cell lymphoma (LBCL). Currently, three CD19-targeted CAR T cell products are approved by the FDA and various other regulators for the treatment of patients with LBCL: axicabtagene ciloleucel, tisagenlecleucel and lisocabtagene maraleucel. Response rates following infusion of these CD19-targeted CAR T cells have been promising; however, approximately half of treated patients show relapse within 2 years. Furthermore, receiving these agents can be associated with serious toxicities, including cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome. In this Review, we summarize the factors associated with the efficacy, including response and survival outcomes, and toxicity of CD19-targeted CAR T cells in pivotal clinical trials and large real-world datasets describing the outcomes of patients with LBCL who received treatment with these products. CD19-targeted CAR T cells have transformed the management of patients with relapsed and/or refractory large B cell lymphoma, and these therapies are increasingly being administered as earlier-line therapies. Nonetheless, the prognosis of these patients is often difficult to predict, with various prospective and real-world studies suggesting that a wide range of factors are associated with treatment outcomes. In this Review, the authors summarize these various associations as well as their implications for patient selection and management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


