CsPbBr3纳米晶体在流动中经历阶跃温度变化:形态演变和光学性质
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于无机钙钛矿纳米晶体具有显著的量产适应性,其微尺度流动策略得到了迅速发展。然而,现有的微流控系统属于单级热反应器,无法像热注射法那样在数控制备过程中实现温度调节,以获得有利的结晶和生长温度。本文设计了两级微流控系统,采用两个加热反应模块,实现了CsPbBr3 NCs在流动中双反应温度的生长。由于第一步前驱体结合温度较低而形成的纳米血小板在第二次加热反应中大部分转化为立方纳米碳,提高了CsPbBr3纳米碳的稳定性。此外,混合的形貌可能阻碍了放大自发发射(ASE)性能,因此均匀的立方纳米(平均尺寸为18.3 nm)在室温下的ASE阈值较低,为4.6 μJ cm-2。此外,该体系有效地避免了PbBr2前驱体在高温下的沉淀,从而在制备温度提高到250℃时成功制备了更大尺寸的纳米碳管(28.2 nm)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
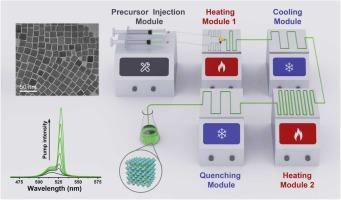
CsPbBr3 nanocrystals experience step temperature variation in flow: Morphology evolution and optical properties
Due to their remarkable suitability for mass production, micro-scale flow strategies for inorganic perovskite nanocrystals (NCs) are rapidly developed. However, the existing microfluidic systems belong to single-stage thermal reactors and cannot achieve temperature adjustment during NC preparation like the hot-injection method to attain beneficial crystallization and growth temperatures. In this work, we designed two-stage microfluidic system with two heating reaction modules to carry out the growth of CsPbBr3 NCs experiencing dual reaction temperatures in flow. The nanoplatelets formed due to lower precursor combination temperatures in the first stage are mostly transformed into cuboidal NCs by a second heating reaction, improving the stability of the CsPbBr3 NCs. Furthermore, the mixed morphology probably impedes the amplified spontaneous emission (ASE) performance, so uniform cuboidal NCs (average size of 18.3 nm) have a lower ASE threshold of 4.6 μJ cm−2 at room temperature. Additionally, this system effectively avoids the precipitation of PbBr2 precursor at elevated temperatures, thus enabling the successful fabrication of larger-sized NCs (28.2 nm) upon raising the preparation temperature to 250 °C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


