在异糖酸盐和邻苯二甲酸盐存在下,波特兰铁矿对放射性核素的保留以及三元配合物的可能作用
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
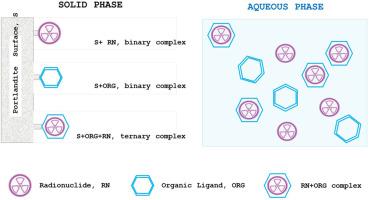
本研究评估了在两种有机配体(异糖酸酯(ISA)和邻苯二甲酸酯(PHTH))存在下,各种放射性核素(RN),特别是63Ni、233U、152Eu和238Pu在硅酸盐上的保留机制。ORG对固相吸收RN的潜在负面影响可能来自分析的两个主要机制:(a) ORG的竞争性吸附或(b)在水相中形成RN-ORG络合物。ISA在硅酸盐上的吸附最大对数(Kd)值为2.1 mL g−1,当ISA总浓度超过1·10−3 m时,对数(Kd)值下降。使用Thermochimie 12a热力学数据库计算的水相RN形态显示,只有在ISA存在的情况下才会形成RN- org复合物。在ISA或PHTH存在下观察到的RN吸附行为与这些发现一致。与PHTH相比,ISA的计算吸附减少因子(SRFs)显著高于PHTH,特别是152Eu和238Pu。然而,ISA对RN保留的有害影响比地球化学模型预测的要低几个数量级,有时,ORG的存在(特别是在低浓度下)增加了RN的吸收,这表明形成了RN + ORG + portlandite三元配合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Radionuclide retention by portlandite in the presence of isosaccharinate and phthalate and possible role of ternary complexes
This research evaluates the retention mechanisms for various radionuclides (RN) specifically, 63Ni, 233U, 152Eu and 238Pu, on portlandite, in the presence of two organic ligands (ORG): isosaccharinate (ISA) and phthalate (PHTH).
The potential negative impact of ORG on RN uptake by the solid phase may arise from two main mechanisms that were analysed: (a) the competitive adsorption of ORG or (b) the formation of RN-ORG complexes in the aqueous phase.
The sorption of ISA on portlandite exhibited a maximum log(Kd) value of 2.1 mL g−1, decreasing when the total ISA concentration exceeded 1·10−3 M. No sorption of PHTH was detected. Aqueous RN speciation, calculated using the Thermochimie 12a thermodynamic database, revealed formation of RN-ORG complexes only in the presence of ISA.
The observed RN sorption behavior in the presence of ISA or PHTH aligned with these findings. The calculated sorption reduction factors (SRFs) were significantly higher for ISA compared to PHTH, particularly for 152Eu and 238Pu. However, the detrimental effect of ISA on RN retention was orders of magnitude lower than the predicted by geochemical modelling and, sometimes, the presence of ORG (especially at low concentrations) increased RN sorption, suggesting the formation of RN + ORG + portlandite ternary complexes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


