通过改变Cu-Pd双金属催化剂的组成来调整电化学CO2还原:实验和理论研究
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在全球变暖的背景下,电化学CO2还原(eCO2RR)为通过生产增值产品实现净零碳排放提供了一条有希望的途径。本研究研究了铜钯(Cu-Pd)双金属催化剂的eCO2RR,重点研究了不同催化剂组成的产物分布。合成了Cu-Pd催化剂,并对其结晶度、结构、织构和形貌进行了表征。Cu-Pd摩尔比(1:1,2:1,1:2,3:1)在−0.6 ~−1.6 V / RHE条件下反应1 h,可得到多种产物。在−1.6 V下,1:1的Cu-Pd比例对甲酸酯的法拉第效率(FE)达到91%,而2:1和1:2的Cu-Pd比例在−1.4 V下产生乙酸酯的法拉第效率为58%和35%。在−1.6 V下,以3:1的比例得到38% FE的甲醇。XPS分析表明,金属氧化物/金属界面抑制了氢的析出,促进了反应中间体的生成。量子力学计算证实了实验结果,强调了潜在依赖的产物选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
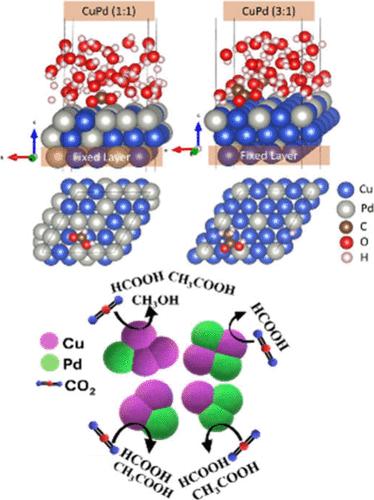
Tuning Electrochemical CO2 Reduction through Variation in Composition of the Cu–Pd Bimetallic Catalyst: Experimental and Theoretical Investigations
In the context of global warming, electrochemical reduction of CO2 (eCO2RR) offers a promising route to achieve net-zero carbon emissions by producing value-added products. This study investigates copper–palladium (Cu–Pd) bimetallic catalysts for the eCO2RR, focusing on product distribution by varying catalyst composition. Cu–Pd catalysts were synthesized and characterized for crystallinity, structure, texture, and morphology. Reactions conducted with Cu–Pd molar ratios (1:1, 2:1, 1:2, 3:1) at −0.6 to −1.6 V vs RHE for 1 h yielded diverse products. A 1:1 Cu–Pd ratio achieved 91% Faradaic efficiency (FE) for formate at −1.6 V, while 2:1 and 1:2 ratios produced acetate with FEs of 58% and 35% at −1.4 V. A 3:1 ratio led to methanol with 38% FE at −1.6 V. XPS analysis revealed the metal oxide/metal interface suppressed hydrogen evolution while facilitating reaction intermediates. Quantum mechanical calculations corroborated experimental results, highlighting potential-dependent product selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


