Ru-S 复合物催化的 N-杂环烯 C-H 硅烷化和硼酸化的机理透视:不同的键合相互作用
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
n -杂芳烃的硅基化和硼基化是制备有机合成中关键组成部分的重要过程。Ru - s配合物1 [(PEt3)Ru(DmpS)]+ (DmpS = 2,6-基苯基硫代酸酯)催化n -杂芳烃的C-H硅基化和硼基化。在此,我们进行了密度泛函研究,探讨了1-甲基吲哚在氢硅烷和二氧氢硼烷(HBpin)和二烷基氢硼烷(9BBN)催化下的C-H硅化反应机制。该机理包括四个主要步骤:(i) Si-H / B-H活化,(ii)硅基/硼基转移到1-甲基吲哚,(iii)质子提取以产生硅基化/硼化产物,(iv) H2消除以再生配合物1。速率决定步骤是硅基/硼基转移。值得注意的是,在B-H激活后,HBpin的B-H键完全断裂,而9BBN的B-H键保持部分完整。此外,Si-H和B-H活化并没有形成硅/硼离子,而是形成了不同的Si-H / B-H活化配合物:(i)氢硅烷和HBpin的硫代硅烷/硫代硼烷支持的Ru-H配合物;(ii) 9BBN的三中心双电子Ru-H - b配合物。键相互作用的差异影响了硅基/硼基转移中的能量势垒。这些电子结构的深入研究为设计n -杂芳烃C-H硅基化和硼基化的金属配体协同催化剂提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
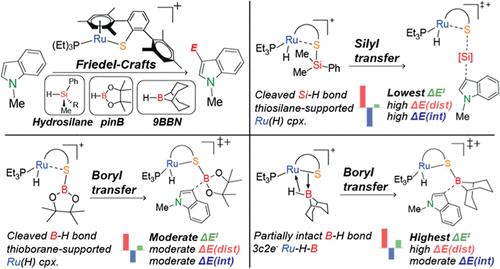
Mechanistic Insights into Ru–S Complex-Catalyzed C–H Silylation and Borylation of N-Heteroarene: Distinct Bonding Interactions
The silylation and borylation of N-heteroarenes are essential processes for preparing key building blocks in organic synthesis. The Ru–S complex 1, [(PEt3)Ru(DmpS)]+ (DmpS = 2,6-dimesitylphenyl thiolate), catalyzes both C–H silylation and borylation of N-heteroarenes. Herein, we performed a density functional study to investigate the mechanisms of 1 catalyzed C–H silylation of 1-methylindole using hydrosilanes and C–H borylation using dialkoxyhydroborane (HBpin) and dialkylhydroborane (9BBN). The mechanism involves four main steps: (i) Si–H/B–H activation, (ii) silyl/boryl transfer to 1-methylindole, (iii) proton abstraction to yield the silylated/borylated product, and (iv) H2 elimination to regenerate complex 1. The rate-determining step is silyl/boryl transfer. Notably, upon B–H activation, the B–H bond of HBpin is fully cleaved, while the B–H bond of 9BBN remains partially intact. Moreover, instead of forming silylium/borenium ions, the Si–H and B–H activations lead to distinct Si–H/B–H-activated complexes: (i) thiosilane/thioborane-supported Ru–H complexes for hydrosilane and HBpin and (ii) a three-center two-electron Ru–H–B complex for 9BBN. Differences in bonding interactions affect the energy barriers in the silyl/boryl transfer. Insights into these electronic structures provide a foundation for designing metal–ligand cooperative catalysts for C–H silylation and borylation of N-heteroarenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


