Cu-ZnO-Al2O3催化剂连续加氢糠醛生产生物燃料的增产及技术经济分析
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在寻找替代燃料的道路上,2-甲基呋喃是一个完美的绿色解决方案。本文首次报道了在工业Cu-ZnO-Al2O3 (CZA)催化剂上连续生产2-甲基呋喃(2-MF),这是一种从生物质衍生的糠醛(FFA)中提取的可持续生物燃料。改进的共沉淀法为合成的催化剂提供了均匀分散的晶体结构,并实现了预期的铜(Cu)负载。不同的Cu负载影响催化行为和活性。因此,我们详细研究了两种Cu负载量分别为9.8和4.7%的CZA催化剂,分别记为C1和C2。通过XRD、N2吸附、H2-TPR、NH3-TPD、XPS、ICP-MS和TEM对催化剂进行了表征。值得注意的是,所制备的催化剂具有平衡的酸位和介孔,高的比表面积和孔体积,以及更好地控制纳米颗粒的大小,从而促进了催化活性。TEM和H2-TPR研究表明铜的分散性较好。XPS还原后仍存在Cu2+和Cu +,证明了所合成催化剂的有效性。此外,TGA还表明了CZA催化剂的稳定性。为了了解催化活性和选择性,在固定床不锈钢填充床反应器中进行了研究。较好的理化性能使得FFA转化率高达33.8%,对2-MF的选择性高达99.6%。在反应过程中没有产生副产物,否则用连续法是不可能的。与现有文献比较,发现CZA催化剂具有优异的催化性能。研究了糠醛加氢制2-甲基呋喃的反应动力学,发现反应级数高,活化能为61.2 kJ/mol。从180°C到220°C,速率常数k明显符合阿伦尼乌斯定律。此外,反应动力学的评价也表明,没有环氢化和脱碳产物,这是很难实现的。最后,该工艺具有显著的经济可行性,2-甲基呋喃的最低平均生产成本为173,068.16美元/吨,总能源效率为78.32%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
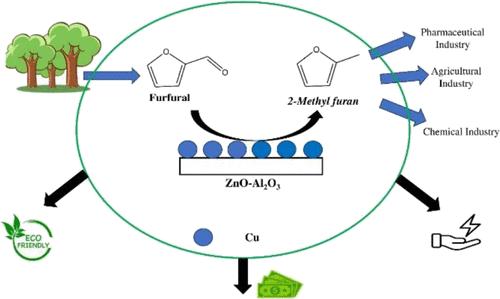
Enhanced Production and Techno-Economic Analysis of Sustainable Biofuel Production via Continuous Hydrogenation of Furfural Using the Cu–ZnO–Al2O3 Catalyst
2-Methylfuran is a perfect green solution on the pathway of finding alternative fuels. We report here for the first time the continuous production of 2-methylfuran (2-MF), a sustainable biofuel from biomass-derived furfural (FFA), over an industrial Cu–ZnO–Al2O3 (CZA) catalyst. The modified coprecipitation method provides a uniformly dispersed crystalline structure to the synthesized catalysts, along with intended copper (Cu) loading achievement. Different Cu loadings affect the catalytic behavior and activity. Hence, CZA catalysts with two Cu loadings of 9.8 and 4.7% were studied in detail, denoted as C1 and C2, respectively. The catalysts were characterized via XRD, N2 adsorption, H2-TPR, NH3-TPD, XPS, ICP-MS, and TEM. Remarkably, the prepared catalysts demonstrate balanced acid sites with mesopores, a high surface area and pore volume, and better controlled nanoparticle size promoting catalytic activity. TEM and H2-TPR studies reveal a better Cu dispersion. Existence of Cu2+ and Cu + even after reduction by XPS study proves the efficiency of the synthesized catalysts. Furthermore, TGA indicates the stability of CZA catalysts. To understand catalytic activity and selectivity, the investigation was carried out in a packed-bed fixed-bed stainless steel reactor. Better physiochemical properties result in high FFA conversion of 33.8% and selectivity of 99.6% for 2-MF. No side products were formed during reaction otherwise improbable via the continuous method. Compared with available literature, the CZA catalyst was found to exhibit superior catalytic performance. The reaction kinetics of furfural hydrogenation to 2-methylfuran was investigated, and it was found that the reaction order is high, and the activation energy was 61.2 kJ/mol. The rate constant k clearly obeyed the Arrhenius law from 180 to 220 °C. In addition, evaluation of reaction kinetics also indicated the absence of ring hydrogenation and decarbonylation products, which is difficult to achieve. Finally, the process shows significant economic viability, which resulted in the minimum levelized production cost for 2-methylfuran of 173,068.16 $/ton with 78.32% overall energy efficiency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


