调节 HZSM-5 沸石中锌位点的反应活性以实现轻烷烃活化
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
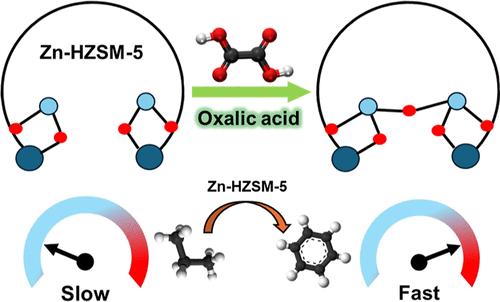
双官能团金属改性沸石已广泛应用于各种催化转化,如脱氢芳构化(DHA)、脱氢、加氢裂化和加氢异构化,其催化活性主要依赖于沸石孔内金属位的结构。本研究表明,在水热或溶剂热条件下,用草酸(OA)溶液处理Zn交换的HZSM-5,形成Zn oxalate@HZSM-5作为活性Zn位点的中间体,可以增强HZSM-5中Zn位点对轻烷烃活化/DHA的反应活性。具体来说,与未经处理的相同样品相比,经过oa处理的Zn-ZSM-5催化剂在丙烷DHA中的活性提高了约460%。我们认为,在轻烷烃活化过程中,锌位点的反应活性增强是因为形成了聚合的草酸锌片段,在h2辅助活化过程中调整了锌位点的配位结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tuning the Reactivity of Zn Sites in HZSM-5 Zeolite for Light Alkane Activation
Bifunctional metal-modified zeolites have been widely employed for various catalytic transformations such as dehydroaromatization (DHA), dehydrogenation, hydrocracking, and hydroisomerization, and their catalytic activity mainly relies on the structure of the metal sites inside the pores of the zeolites. Here, we show that the reactivity of Zn sites (in HZSM-5) for light alkane activation/DHA can be enhanced by treating Zn-exchanged HZSM-5 with an oxalic acid (OA) solution under hydrothermal or solvothermal conditions to form Zn oxalate@HZSM-5 as an intermediate to the active Zn sites. Specifically, the activity of the OA-treated Zn-ZSM-5 catalyst in propane DHA was enhanced by about 460% in contrast to the same sample without treatment. We suggest that the reactivity of Zn sites for light alkane activation was enhanced because of the formation of polymeric Zn oxalate segments, which tuned the coordination structure of the Zn sites upon H2-assisted activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


