在 2-Me-THF 中进行光化学有氧磺酰化-环化-硒化反应,生成吲哚融合的中型 N-杂环
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
吲哚融合的中型二氮杂卓酮类化合物是许多高价值药物中的重要结构基团。为了构建这些具有挑战性的分子骨架,以往的方法主要是借助过渡金属(Pd、Rh、Ru)催化的氧化 C-H 偶联,通过 [5+2] 环化策略来实现。在此,我们报告了一种新型的可见光诱导磺酰化-环化-硒化反应,该反应以生物质原料 2-Me-THF 为介质,可快速构建高官能度的吲哚融合中型二氮杂卓酮类化合物。根据所研究的机理,提出了一条亲电自由基磺酰化-环化和随后的有氧亲电 C-3 硒化路线。本文章由计算机程序翻译,如有差异,请以英文原文为准。
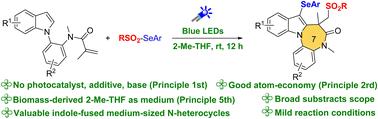
Photochemical aerobic sulfonylation–cyclization–selenylation to indole-fused medium-sized N-heterocycles in 2-Me-THF
Indole-fused medium-sized diazepinones are privileged structural motifs found in many high-value pharmaceuticals. To construct these challenging molecular skeletons, previous methods are mainly achieved by [5+2] annulation strategies with the aid of transition metal (Pd, Rh, Ru) catalyzed oxidative C–H coupling. Herein, we report a novel visible-light-induced sulfonylation–cyclization–selenylation reaction for the rapid construction of highly functionalized indole-fused medium-sized diazepinones with biomass feedstock 2-Me-THF as the medium. Based on the mechanism studied, an electrophilic radical sulfonylation–cyclization and subsequent aerobic electrophilic C-3 selenylation route is proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


